The frequency of polyploid speciation in vascular plants - PubMed (original) (raw)
The frequency of polyploid speciation in vascular plants
Troy E Wood et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Since its discovery in 1907, polyploidy has been recognized as an important phenomenon in vascular plants, and several lines of evidence indicate that most, if not all, plant species ultimately have a polyploid ancestry. However, previous estimates of the frequency of polyploid speciation suggest that the formation and establishment of neopolyploid species is rare. By combining information from the botanical community's vast cytogenetic and phylogenetic databases, we establish that 15% of angiosperm and 31% of fern speciation events are accompanied by ploidy increase. These frequency estimates are higher by a factor of four than earlier estimates and lead to a standing incidence of polyploid species within genera of 35% (n = 1,506). Despite this high incidence, we find no direct evidence that polyploid lines, once established, enjoy greater net species diversification. Thus, the widespread occurrence of polyploid taxa appears to result from the substantial contribution of polyploidy to cladogenesis, but not from subsequent increases in diversification rates of polyploid lines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Polyploid incidence and speciation frequencies across major groups of vascular plants. Polyploid speciation frequencies are the fractions of branching events that were accompanied by a ploidy shift across the studied phylogenetic trees for each group. The speciation frequencies reported here are based on an irreversible model of polyploid evolution. A binomial standard error follows each incidence and frequency estimate. See
Fig. S1
for a diagrammatic explanation of estimation methods for polyploid speciation frequencies. Phylogenetic hypothesis/timescale modified from (13), and based on clades defined in refs. –; clade species richness from refs. and . The Higher Monocots are represented by Arecales, Commelinales, Poales, Proteales, Zingiberales; the Basal Monocots by Alistmatales, Asparagales, Dioscoreales, Liliales, Pandanales.
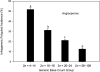
Fig. 2.
Dependence of infrageneric polyploid incidence on the minimum number of chromosomes reported for angiosperm genera (generic base count). Shown are mean percentages (± SE) of polyploid species within genera. Means with different letters are significantly different [P < 0.05 when evaluated within a logistic model and with nonparametric (rank sums) tests]. (N (l − r) = 320, 323, 370, 354).
Similar articles
- Polyploid incidence and evolution.
Otto SP, Whitton J. Otto SP, et al. Annu Rev Genet. 2000;34:401-437. doi: 10.1146/annurev.genet.34.1.401. Annu Rev Genet. 2000. PMID: 11092833 Review. - Karyotypic changes through dysploidy persist longer over evolutionary time than polyploid changes.
Escudero M, Martín-Bravo S, Mayrose I, Fernández-Mazuecos M, Fiz-Palacios O, Hipp AL, Pimentel M, Jiménez-Mejías P, Valcárcel V, Vargas P, Luceño M. Escudero M, et al. PLoS One. 2014 Jan 9;9(1):e85266. doi: 10.1371/journal.pone.0085266. eCollection 2014. PLoS One. 2014. PMID: 24416374 Free PMC article. - Impact of whole-genome duplication events on diversification rates in angiosperms.
Landis JB, Soltis DE, Li Z, Marx HE, Barker MS, Tank DC, Soltis PS. Landis JB, et al. Am J Bot. 2018 Mar;105(3):348-363. doi: 10.1002/ajb2.1060. Epub 2018 May 2. Am J Bot. 2018. PMID: 29719043 - Polyploid phylogenetics.
Rothfels CJ. Rothfels CJ. New Phytol. 2021 Apr;230(1):66-72. doi: 10.1111/nph.17105. Epub 2021 Jan 25. New Phytol. 2021. PMID: 33491778 Review. - Polyploid species rely on vegetative reproduction more than diploids: a re-examination of the old hypothesis.
Herben T, Suda J, Klimešová J. Herben T, et al. Ann Bot. 2017 Aug 1;120(2):341-349. doi: 10.1093/aob/mcx009. Ann Bot. 2017. PMID: 28334206 Free PMC article.
Cited by
- Genetic Variation, Polyploidy, Hybridization Influencing the Aroma Profiles of Rosaceae Family.
Chen X, Zhang Y, Tang W, Zhang G, Wang Y, Yan Z. Chen X, et al. Genes (Basel). 2024 Oct 18;15(10):1339. doi: 10.3390/genes15101339. Genes (Basel). 2024. PMID: 39457463 Free PMC article. Review. - Origin and evolution of a new tetraploid mangrove species in an intertidal zone.
Feng H, Banerjee AK, Guo W, Yuan Y, Duan F, Ng WL, Zhao X, Liu Y, Li C, Liu Y, Li L, Huang Y. Feng H, et al. Plant Divers. 2024 Apr 26;46(4):476-490. doi: 10.1016/j.pld.2024.04.007. eCollection 2024 Jul. Plant Divers. 2024. PMID: 39280974 Free PMC article. - Integrative analyses of long and short-read RNA sequencing reveal the spliced isoform regulatory network of seedling growth dynamics in upland cotton.
Shahzad K, Zhang M, Mubeen I, Zhang X, Guo L, Qi T, Feng J, Tang H, Qiao X, Wu J, Xing C. Shahzad K, et al. Funct Integr Genomics. 2024 Sep 4;24(5):156. doi: 10.1007/s10142-024-01420-0. Funct Integr Genomics. 2024. PMID: 39230785 - The interplay between climatic niche evolution, polyploidy and reproductive traits explains plant speciation in the Mediterranean Basin: a case study in Centaurium (Gentianaceae).
Valdés-Florido A, Valcárcel V, Maguilla E, Díaz-Lifante Z, Andrés-Camacho C, Zeltner L, Coca-de-la-Iglesia M, Medina NG, Arroyo J, Escudero M. Valdés-Florido A, et al. Front Plant Sci. 2024 Aug 9;15:1439985. doi: 10.3389/fpls.2024.1439985. eCollection 2024. Front Plant Sci. 2024. PMID: 39184574 Free PMC article.
References
- Lutz AM. A preliminary note on the chromosomes of Oenothera Lamarckiana and one of its mutants, O gigas. Science. 1907;26:151–152. - PubMed
- Stebbins GL. Variation and Evolution in Plants. New York: Columbia Univ Press; 1950.
- Grant V. Plant Speciation. New York: Columbia Univ Press; 1981.
- Masterson J. Stomatal size in fossil plants—evidence for polyploidy in majority of angiosperms. Science. 1994;264:1759–1763. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources