Bub1 and Bub3 promote the conversion from monopolar to bipolar chromosome attachment independently of shugoshin - PubMed (original) (raw)
Bub1 and Bub3 promote the conversion from monopolar to bipolar chromosome attachment independently of shugoshin
Hanna Windecker et al. EMBO Rep. 2009 Sep.
Abstract
The eukaryotic spindle assembly checkpoint (SAC) delays anaphase in the presence of chromosome attachment errors. Bub3 has been reported to be required for SAC activity in all eukaryotes examined so far. We find that Bub3, unlike its binding partner Bub1, is not essential for the SAC in fission yeast. As Bub3 is needed for the efficient kinetochore localization of Bub1, and of Mad1, Mad2 and Mad3, this implies that most SAC proteins do not need to be enriched at the kinetochores for the SAC to function. We find that Bub3 is also dispensable for shugoshin localization to the centromeres, which is the second known function of Bub1. Instead, Bub3, together with Bub1, has a specific function in promoting the conversion from chromosome mono-orientation to bi-orientation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
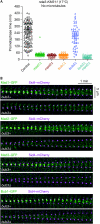
Bub3 is not essential for the SAC, but for the efficient localization of SAC proteins. (A) Cells expressing plo1–GFP and the β-tubulin nda3-KM311 allele were followed by live-cell microscopy at 17°C. The duration of prometaphase was determined by the presence of Plo1–GFP on the SPBs. Circles indicate cells in which the entire prometaphase took place within the recording time. Triangles indicate cells that had not exited prometaphase when recording was stopped; thus the actual time of prometaphase must be longer than this value. Kymographs of exemplary cells are shown in supplementary Fig S1A online. (B) Kymographs of exemplary mitotic cells that were followed by live-cell microscopy at 30°C using Sid4–mCherry to visualize SPBs. A quantitative analysis is shown in supplementary Fig S3 online. GFP, green fluorescent protein; SAC, spindle assembly checkpoint; SPB, spindle pole body.
Figure 2
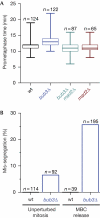
Bub3 is required for correct chromosome segregation. (A) The duration of prometaphase at 30°C in _bub3_Δ, _bub3_Δ _mad2_Δ, _mad2_Δ and wild-type (wt) cells was determined by live-cell microscopy using Plo1–GFP as a marker. In all box-whisker graphs, the lines from top to bottom are: maximum value, 75th percentile, median, 25th percentile and minimum value. (B) Cells carrying _cen2_–GFP and mCherry–atb2(tubulin) were synchronized with HU, released from HU arrest and arrested in mitosis by treatment with the microtubule-destabilizing drug MBC for 3.5 h. After washout of MBC, segregation of chromosome 2 (_cen2_–GFP) was followed by live-cell microscopy at 20°C. Only those cells that were already in mitosis when recording was started were considered. Mis-segregation in unperturbed mitosis was similarly determined by live-cell microscopy at 20°C after HU release. GFP, green fluorescent protein; HU, hydroxyurea.
Figure 3
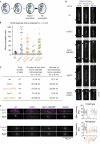
Bub3 and its interaction with Bub1 are required for chromosome bi-orientation in an Sgo2-independent manner. (A–C) Cells expressing GFP–atb2(tubulin) and the kinetochore marker mis6–mCherry were pre-synchronized and treated with MBC as described in Fig 2B. After washout of MBC, segregation of the chromosomes was monitored by using live-cell microscopy at 20°C. (B) Chromosomes that persisted close to an SPB for at least 10 min were followed and the time until they became bi-oriented was determined (open circles). Filled circles indicate chromosomes that apparently never achieved bi-orientation; triangles indicate chromosomes that had failed to achieve bi-orientation by the end of the recording. The total number of cells observed, the number of cells showing mono-oriented chromosomes for at least 10 min and the number of mono-oriented chromosomes are given in (C). (D) Exemplary cells from this experiment. Arrowheads indicate mono-oriented chromosomes. For the _bub3_Δ cell, correction of mono-orientation to bi-orientation can be seen at 33 min. In the _bub1-Δ_GLEBS cell, mono-orientation was never corrected and the cell entered anaphase after 37 min. In the _sgo2_Δ cell, bi-orientation was achieved after about 15 min. The _sgo2_Δ _bub3_Δ cell failed to bi-orient two chromosomes and delayed entry into anaphase for more than 1 h (supplementary Fig S4 online). (E) Living cells from the indicated strains expressing mCherry–atb2(tubulin) and sgo2–GFP were imaged by fluorescence microscopy. For each genotype, two independent strains were used. The maximum signal intensity in the nucleus was determined. Interphase or metaphase cells were identified by the characteristic microtubule signal. _bub3_Δ and bub1_-Δ_GLEBS cells often showed more than one Sgo2–GFP signal in mitosis (supplementary Fig S9 online). GFP, green fluorescent protein; SPB, spindle pole body; wt, wild type.
Figure 4
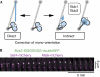
Possible pathways for the correction of chromosome mono-orientation. (A) When chromosomes are clustered close to the SPBs at the beginning of mitosis or when spindles are short, direct capture by a microtubule from the opposite SPB might be the predominant way of correcting mono-orientation (left). When spindles become elongated, further mechanisms might be needed (right). We propose that Bub1 and Bub3 are involved in moving mono-oriented chromosomes closer to the centre of the spindle either by promoting movement along pre-existing kinetochore microtubules or by modulating kinetochore–microtubule attachment of the mono-oriented chromosome to the proximal pole. (B) Kymograph of a _bub3–GFP_-expressing cell treated with MBC after release from HU-arrest as described in Fig 2B. An initially unattached chromosome was captured by microtubules, pulled towards an SPB and subsequently bi-oriented. Bub3–GFP localized to one or both kinetochores of this chromosome until the chromosome had achieved bi-orientation. GFP, green fluorescent protein; HU, hydroxyurea; SPB, spindle pole body.
Figure 5
Mono-orientation of chromosomes in _bub3_Δ cells can be rescued when mitotic spindles are short. (A) Model for the possible mechanism of rescue: when the mitotic spindle is long, microtubules from the opposite spindle pole cannot reach the mono-oriented chromosome, whereas they can if the spindle is short. A second, indirect mechanism of rescue (Fig 4A) is presumably not functional in _bub3_Δ cells. (B–D) Exemplary kymographs of (B) wild-type or (C,D) _bub3_Δ cells carrying _cen2_–GFP and expressing mCherry–atb2(tubulin) from the experiment described in Fig 2B. GFP, green fluorescent protein.
Similar articles
- The Bub1-TPR Domain Interacts Directly with Mad3 to Generate Robust Spindle Checkpoint Arrest.
Leontiou I, London N, May KM, Ma Y, Grzesiak L, Medina-Pritchard B, Amin P, Jeyaprakash AA, Biggins S, Hardwick KG. Leontiou I, et al. Curr Biol. 2019 Jul 22;29(14):2407-2414.e7. doi: 10.1016/j.cub.2019.06.011. Epub 2019 Jun 27. Curr Biol. 2019. PMID: 31257143 Free PMC article. - Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint.
Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, Rappsilber J, Hardwick KG, Millar JB. Shepperd LA, et al. Curr Biol. 2012 May 22;22(10):891-9. doi: 10.1016/j.cub.2012.03.051. Epub 2012 Apr 19. Curr Biol. 2012. PMID: 22521786 Free PMC article. - MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components.
Yamagishi Y, Yang CH, Tanno Y, Watanabe Y. Yamagishi Y, et al. Nat Cell Biol. 2012 Jun 3;14(7):746-52. doi: 10.1038/ncb2515. Nat Cell Biol. 2012. PMID: 22660415 - Kinetochore-microtubule interactions "in check" by Bub1, Bub3 and BubR1: The dual task of attaching and signalling.
Logarinho E, Bousbaa H. Logarinho E, et al. Cell Cycle. 2008 Jun 15;7(12):1763-8. doi: 10.4161/cc.7.12.6180. Epub 2008 Jun 20. Cell Cycle. 2008. PMID: 18594200 Review. - The spindle checkpoint proteins BUB1 and BUBR1: (SLiM)ming down to the basics.
Elowe S, Bolanos-Garcia VM. Elowe S, et al. Trends Biochem Sci. 2022 Apr;47(4):352-366. doi: 10.1016/j.tibs.2022.01.004. Epub 2022 Feb 17. Trends Biochem Sci. 2022. PMID: 35184951 Review.
Cited by
- Mad1 promotes chromosome congression by anchoring a kinesin motor to the kinetochore.
Akera T, Goto Y, Sato M, Yamamoto M, Watanabe Y. Akera T, et al. Nat Cell Biol. 2015 Sep;17(9):1124-33. doi: 10.1038/ncb3219. Epub 2015 Aug 10. Nat Cell Biol. 2015. PMID: 26258632 - Centromere-tethered Mps1 pombe homolog (Mph1) kinase is a sufficient marker for recruitment of the spindle checkpoint protein Bub1, but not Mad1.
Ito D, Saito Y, Matsumoto T. Ito D, et al. Proc Natl Acad Sci U S A. 2012 Jan 3;109(1):209-14. doi: 10.1073/pnas.1114647109. Epub 2011 Dec 19. Proc Natl Acad Sci U S A. 2012. PMID: 22184248 Free PMC article. - Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling.
Primorac I, Weir JR, Chiroli E, Gross F, Hoffmann I, van Gerwen S, Ciliberto A, Musacchio A. Primorac I, et al. Elife. 2013 Sep 24;2:e01030. doi: 10.7554/eLife.01030. Elife. 2013. PMID: 24066227 Free PMC article. - Spindle checkpoint silencing requires association of PP1 to both Spc7 and kinesin-8 motors.
Meadows JC, Shepperd LA, Vanoosthuyse V, Lancaster TC, Sochaj AM, Buttrick GJ, Hardwick KG, Millar JB. Meadows JC, et al. Dev Cell. 2011 Jun 14;20(6):739-50. doi: 10.1016/j.devcel.2011.05.008. Dev Cell. 2011. PMID: 21664573 Free PMC article. - The composition, functions, and regulation of the budding yeast kinetochore.
Biggins S. Biggins S. Genetics. 2013 Aug;194(4):817-46. doi: 10.1534/genetics.112.145276. Genetics. 2013. PMID: 23908374 Free PMC article. Review.
References
- Campbell L, Hardwick KG (2003) Analysis of Bub3 spindle checkpoint function in Xenopus egg extracts. J Cell Sci 116: 617–628 - PubMed
- De Antoni A et al. (2005) The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr Biol 15: 214–225 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous