Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice - PubMed (original) (raw)
. 2009 Sep 1;106(35):14872-7.
doi: 10.1073/pnas.0906587106. Epub 2009 Aug 18.
M A Ravier, A Schraenen, J W M Creemers, R Van de Plas, M Granvik, L Van Lommel, E Waelkens, F Chimienti, G A Rutter, P Gilon, P A in't Veld, F C Schuit
Affiliations
- PMID: 19706465
- PMCID: PMC2736467
- DOI: 10.1073/pnas.0906587106
Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice
K Lemaire et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Zinc co-crystallizes with insulin in dense core secretory granules, but its role in insulin biosynthesis, storage and secretion is unknown. In this study we assessed the role of the zinc transporter ZnT8 using ZnT8-knockout (ZnT8(-/-)) mice. Absence of ZnT8 expression caused loss of zinc release upon stimulation of exocytosis, but normal rates of insulin biosynthesis, normal insulin content and preserved glucose-induced insulin release. Ultrastructurally, mature dense core insulin granules were rare in ZnT8(-/-) beta cells and were replaced by immature, pale insulin "progranules," which were larger than in ZnT8(+/+) islets. When mice were fed a control diet, glucose tolerance and insulin sensitivity were normal. However, after high-fat diet feeding, the ZnT8(-/-) mice became glucose intolerant or diabetic, and islets became less responsive to glucose. Our data show that the ZnT8 transporter is essential for the formation of insulin crystals in beta cells, contributing to the packaging efficiency of stored insulin. Interaction between the ZnT8(-/-) genotype and diet to induce diabetes is a model for further studies of the mechanism of disease of human ZNT8 gene mutations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
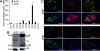
ZnT8 expression is absent in _ZnT8_−/− mice. (A) mRNA expression of the 10 known efflux zinc transporters (Slc30a family) analyzed via microarray (n = 3). (B) ZnT8 protein expression was analyzed via western blots. Please note complete absence of the expected MW for ZnT8 monomers (M) and SDS-resistant dimers (D), * = non-specific protein. (C) Immunohistochemistry of pancreatic sections from _ZnT8_−/− and ZnT8+/+ mice. In ZnT8+/+ mice ZnT8 protein is strongly expressed in all insulin-positive cells and weakly expressed in a minority of glucagon cells. Nuclei are stained blue by 4′,6-diamidino-2-phenylindole (DAPI). (Scale bar, 10 μm.)
Fig. 2.
ZnT8 is required for islet dithizone staining and glucose-regulated granular zinc release from beta cells. (A) Dithizone staining of pancreata from ZnT8+/+ and _ZnT8_−/− mice, 15 min after i.p. injection of the dye. Islets can be seen at the pancreatic surface of ZnT8+/+ mice but not of _ZnT8_−/− mice. (B) Dithizone staining of isolated islets is positive in ZnT8+/+ mice (red color) but negative in _ZnT8_−/− mice. (C) Zinc release from clusters of islet cells imaged by TIRF microscopy during 7 min. Cluster size was 3.7 ± 0.4 vs. 3.8 ± 0.3 cells, for ZnT8+/+ and _ZnT8_−/−, respectively (ns). Medium contained 8 μM FluoZin-3 and exocytosis was stimulated by 15 mM glucose and 1 μM forskolin. One zinc exocytotic event from a ZnT8+/+ four-cell cluster is illustrated, boundaries between cells are shown as dotted lines (left panel). Images were taken every 30 ms in the region delimited by a square (middle panel), and the intensity of fluorescence for this release event is plotted on the right panel. Arrows show fluorescence intensity for the indicated time points illustrated in the middle panel, (D) Quantification of zinc exocytotic events in clusters of ZnT8+/+ and _ZnT8_−/− islet cells. The horizontal line shows the average of 18 clusters from three individual mice.
Fig. 3.
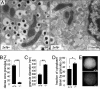
ZnT8 is required for zinc-insulin crystal formation. (A) Transmission electron microscopy of representative ZnT8+/+ and _ZnT8_−/− beta cells (ZnT8+/+, n = 4; _ZnT8_−/−, n = 7). Secretory granules of _ZnT8_−/− beta cells lack the electron dense cores and halo that are typical for wild-type mice. Furthermore, the size of the secretory granules is larger in _ZnT8_−/− mice. (Scale bar, 0.5 μm.) (B) Quantification of % dense core granules in ZnT8+/+ and _ZnT8_−/− beta cells (n = 622 and 740 granules, respectively,), (C and D) Morphometric quantification of granule diameter (n = 126 and 83 granules, respectively), (C) and relative cytoplasmic volume of the granule pool (D) in ZnT8+/+ and _ZnT8_−/− beta cells. Data are mean ± SEM, statistical analysis via unpaired Student's t test: *, P < 0.05; **, P < 0.001; ***, P < 0.0001. (E) Bright light reflection of islolated ZnT8+/+ islets contrasts with dull gray appearance of _ZnT8_−/− islets under a dissection microscope.
Fig. 4.
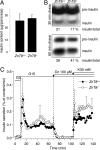
Insulin biosynthesis, processing and release are not affected in _ZnT8_−/− mice. (A) Insulin content from isolated pancreata of _ZnT8_−/− and ZnT8+/+ mice. Data are mean ± SEM, n = 4). (B) Radiometric quantification of pro-insulin synthesis and conversion to insulin. Isolated islets from _ZnT8_−/− and ZnT8+/+ mice were labeled for 30 min with 35S-amino acids and chased for 30 min (upper panel) or 1 h (lower panel) in the presence of 10 mM glucose. Radioactive (pro)-insulin was quantified after immunoprecipitation, gel electrophoresis and autoradiography. Similar amounts of pro-insulin synthesis and conversion were found [30 min (≈20%) and 1 h (≈40%), (n = 3)] in both strains. (C) Glucose-induced insulin release. After overnight culture in 10 mM glucose (G), islets from adult ZnT8+/+ (black circle) and _ZnT8_−/− (open circle) mice were perifused for 30 min with a medium containing 3 mM glucose. When indicated by the arrows, the glucose concentration was increased to 15 mM, the KATP channel opener diazoxide (Dz) was added, and the KCl concentration was increased from 4.8 to 30 mM. Data are mean ± SEM of four experiments.
Fig. 5.
Glucose and insulin tolerance in ZnT8+/+ and _ZnT8_−/− mice fed a control diet. ZnT8+/+ (black circles) and _ZnT8_−/− (open circles) mice were given a control chow and were tested between 6 and 52 weeks of age. Blood glucose levels were measured at the indicated time points after i.p. injection of 2.5 mg glucose/g BW glucose in overnight (16 h) fasted mice aged 6 weeks (A), 12 weeks (B), 25 weeks (C), and 1 year (D). (E) Insulin tolerance test after injection of 0.75 mU/g BW insulin i.p. in 6 h fasted mice aged 12 weeks, expressed as % over baseline. Data are mean ± SEM, statistical analysis via unpaired Student's t test: *, P ≤ 0.05.
Similar articles
- Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants.
Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, Taneja TK, da Silva Xavier G, Libert S, Froguel P, Scharfmann R, Stetsyuk V, Ravassard P, Parker H, Gribble FM, Reimann F, Sladek R, Hughes SJ, Johnson PR, Masseboeuf M, Burcelin R, Baldwin SA, Liu M, Lara-Lemus R, Arvan P, Schuit FC, Wheeler MB, Chimienti F, Rutter GA. Nicolson TJ, et al. Diabetes. 2009 Sep;58(9):2070-83. doi: 10.2337/db09-0551. Epub 2009 Jun 19. Diabetes. 2009. PMID: 19542200 Free PMC article. - Molecular Genetic Regulation of Slc30a8/ZnT8 Reveals a Positive Association With Glucose Tolerance.
Mitchell RK, Hu M, Chabosseau PL, Cane MC, Meur G, Bellomo EA, Carzaniga R, Collinson LM, Li WH, Hodson DJ, Rutter GA. Mitchell RK, et al. Mol Endocrinol. 2016 Jan;30(1):77-91. doi: 10.1210/me.2015-1227. Epub 2015 Nov 19. Mol Endocrinol. 2016. PMID: 26584158 Free PMC article. - Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion.
Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, Chimienti F, Gaisano HY, Rutter GA, Wheeler MB. Wijesekara N, et al. Diabetologia. 2010 Aug;53(8):1656-68. doi: 10.1007/s00125-010-1733-9. Epub 2010 Apr 28. Diabetologia. 2010. PMID: 20424817 Free PMC article. - Zinc transporter 8 (ZnT8) and β cell function.
Davidson HW, Wenzlau JM, O'Brien RM. Davidson HW, et al. Trends Endocrinol Metab. 2014 Aug;25(8):415-24. doi: 10.1016/j.tem.2014.03.008. Epub 2014 Apr 18. Trends Endocrinol Metab. 2014. PMID: 24751356 Free PMC article. Review. - Different role of zinc transporter 8 between type 1 diabetes mellitus and type 2 diabetes mellitus.
Yi B, Huang G, Zhou Z. Yi B, et al. J Diabetes Investig. 2016 Jul;7(4):459-65. doi: 10.1111/jdi.12441. Epub 2016 Jan 9. J Diabetes Investig. 2016. PMID: 27181765 Free PMC article. Review.
Cited by
- Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance.
Huang L, Kirschke CP, Lay YA, Levy LB, Lamirande DE, Zhang PH. Huang L, et al. J Biol Chem. 2012 Sep 28;287(40):33883-96. doi: 10.1074/jbc.M111.309666. Epub 2012 Aug 1. J Biol Chem. 2012. PMID: 22854958 Free PMC article. - Light scattering as an intrinsic indicator for pancreatic islet cell mass and secretion.
Ilegems E, van Krieken PP, Edlund PK, Dicker A, Alanentalo T, Eriksson M, Mandic S, Ahlgren U, Berggren PO. Ilegems E, et al. Sci Rep. 2015 Jun 1;5:10740. doi: 10.1038/srep10740. Sci Rep. 2015. PMID: 26030284 Free PMC article. - Diabetes mellitus due to the toxic misfolding of proinsulin variants.
Weiss MA. Weiss MA. FEBS Lett. 2013 Jun 27;587(13):1942-50. doi: 10.1016/j.febslet.2013.04.044. Epub 2013 May 10. FEBS Lett. 2013. PMID: 23669362 Free PMC article. Review. - Liquid-liquid phase separation facilitates the biogenesis of secretory storage granules.
Parchure A, Tian M, Stalder D, Boyer CK, Bearrows SC, Rohli KE, Zhang J, Rivera-Molina F, Ramazanov BR, Mahata SK, Wang Y, Stephens SB, Gershlick DC, von Blume J. Parchure A, et al. J Cell Biol. 2022 Dec 5;221(12):e202206132. doi: 10.1083/jcb.202206132. Epub 2022 Sep 29. J Cell Biol. 2022. PMID: 36173346 Free PMC article. - Role of zinc in human islet amyloid polypeptide aggregation.
Brender JR, Hartman K, Nanga RP, Popovych N, de la Salud Bea R, Vivekanandan S, Marsh EN, Ramamoorthy A. Brender JR, et al. J Am Chem Soc. 2010 Jul 7;132(26):8973-83. doi: 10.1021/ja1007867. J Am Chem Soc. 2010. PMID: 20536124 Free PMC article.
References
- Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. - PubMed
- Dodson G, Steiner D. The role of assembly in insulin's biosynthesis. Curr Opin Struct Biol. 1998;8:189–194. - PubMed
- Huang XF, Arvan P. Intracellular transport of proinsulin in pancreatic beta-cells. Structural maturation probed by disulfide accessibility. J Biol Chem. 1995;270:20417–20423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases