A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms - PubMed (original) (raw)
Review
A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms
Suneel S Apte. J Biol Chem. 2009.
Abstract
Together with seven ADAMTS-like proteins, the 19 mammalian ADAMTS proteases constitute a superfamily. ADAMTS proteases are secreted zinc metalloproteases whose hallmark is an ancillary domain containing one or more thrombospondin type 1 repeats. ADAMTS-like proteins resemble ADAMTS ancillary domains and lack proteolytic activity. Vertebrate expansion of the superfamily reflects emergence of new substrates, duplication of proteolytic activities in new contexts, and cooperative functions of the duplicated genes. ADAMTS proteases are involved in maturation of procollagen and von Willebrand factor, as well as in extracellular matrix proteolysis relating to morphogenesis, angiogenesis, ovulation, cancer, and arthritis. New insights into ADAMTS mechanisms indicate significant regulatory roles for ADAMTS ancillary domains, propeptide processing, and glycosylation. ADAMTS-like proteins appear to have regulatory roles in the extracellular matrix.
Figures
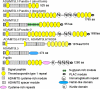
FIGURE 1.
Mammalian ADAMTS proteases. The domain backbone shared by each ADAMTS protease is shown at the top. The unique structure of each ADAMTS protease C-terminal to the backbone is indicated on the right, and the key to these modules is located on the left. Some clades are named according to structural or functional characteristics that best define them; clades without a known function or a defining characteristic are not named. The proteoglycanases constitute a superclade comprising ADAMTS proteases with different domain structure. The figure is based on reference sequences obtained from GenBankTM. PLAC,
p
rotease and
lac
unin module.
FIGURE 2.
Mammalian ADAMTSL proteins. The domain structure of each ADAMTSL is shown according to the key at the bottom. The two forms of ADAMTSL1 shown are splice variants, and the long form composes a clade with ADAMTSL3. ADAMTSL4 and ADAMTSL6 compose a distinct clade in which TSR1 is split by an insertion. The figure is based on reference sequences obtained from GenBankTM. aa, amino acids. PLAC,
p
rotease and
lac
unin module.
Similar articles
- A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family.
Apte SS. Apte SS. Int J Biochem Cell Biol. 2004 Jun;36(6):981-5. doi: 10.1016/j.biocel.2004.01.014. Int J Biochem Cell Biol. 2004. PMID: 15094112 Review. - ADAMTS: a novel family of extracellular matrix proteases.
Tang BL. Tang BL. Int J Biochem Cell Biol. 2001 Jan;33(1):33-44. doi: 10.1016/s1357-2725(00)00061-3. Int J Biochem Cell Biol. 2001. PMID: 11167130 Review. - The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family.
Kelwick R, Desanlis I, Wheeler GN, Edwards DR. Kelwick R, et al. Genome Biol. 2015 May 30;16(1):113. doi: 10.1186/s13059-015-0676-3. Genome Biol. 2015. PMID: 26025392 Free PMC article. Review. - Crystal structures of human ADAMTS-1 reveal a conserved catalytic domain and a disintegrin-like domain with a fold homologous to cysteine-rich domains.
Gerhardt S, Hassall G, Hawtin P, McCall E, Flavell L, Minshull C, Hargreaves D, Ting A, Pauptit RA, Parker AE, Abbott WM. Gerhardt S, et al. J Mol Biol. 2007 Nov 2;373(4):891-902. doi: 10.1016/j.jmb.2007.07.047. Epub 2007 Aug 2. J Mol Biol. 2007. PMID: 17897672 - A disintegrin-like and metalloprotease domain containing thrombospondin type 1 motif-like 5 (ADAMTSL5) is a novel fibrillin-1-, fibrillin-2-, and heparin-binding member of the ADAMTS superfamily containing a netrin-like module.
Bader HL, Wang LW, Ho JC, Tran T, Holden P, Fitzgerald J, Atit RP, Reinhardt DP, Apte SS. Bader HL, et al. Matrix Biol. 2012 Sep-Oct;31(7-8):398-411. doi: 10.1016/j.matbio.2012.09.003. Epub 2012 Sep 23. Matrix Biol. 2012. PMID: 23010571 Free PMC article.
Cited by
- Screening ADAMTS10 in dog populations supports Gly661Arg as the glaucoma-causing variant in beagles.
Kuchtey J, Kunkel J, Esson D, Sapienza JS, Ward DA, Plummer CE, Gelatt KN, Kuchtey RW. Kuchtey J, et al. Invest Ophthalmol Vis Sci. 2013 Mar 13;54(3):1881-6. doi: 10.1167/iovs.12-10796. Invest Ophthalmol Vis Sci. 2013. PMID: 23422823 Free PMC article. - A disintegrin and metalloprotease with thrombospondin type I motif 7: a new protease for connective tissue growth factor in hepatic progenitor/oval cell niche.
Pi L, Jorgensen M, Oh SH, Protopapadakis Y, Gjymishka A, Brown A, Robinson P, Liu C, Scott EW, Schultz GS, Petersen BE. Pi L, et al. Am J Pathol. 2015 Jun;185(6):1552-63. doi: 10.1016/j.ajpath.2015.02.008. Epub 2015 Apr 2. Am J Pathol. 2015. PMID: 25843683 Free PMC article. - Transcriptional regulation by the Wilms tumor protein, Wt1, suggests a role of the metalloproteinase Adamts16 in murine genitourinary development.
Jacobi CL, Rudigier LJ, Scholz H, Kirschner KM. Jacobi CL, et al. J Biol Chem. 2013 Jun 28;288(26):18811-24. doi: 10.1074/jbc.M113.464644. Epub 2013 May 9. J Biol Chem. 2013. PMID: 23661704 Free PMC article. - Matrix-dependent perturbation of TGFβ signaling and disease.
Doyle JJ, Gerber EE, Dietz HC. Doyle JJ, et al. FEBS Lett. 2012 Jul 4;586(14):2003-15. doi: 10.1016/j.febslet.2012.05.027. Epub 2012 May 26. FEBS Lett. 2012. PMID: 22641039 Free PMC article. Review. - Biochemical composition and turnover of the extracellular matrix of the normal and degenerate intervertebral disc.
Sivan SS, Hayes AJ, Wachtel E, Caterson B, Merkher Y, Maroudas A, Brown S, Roberts S. Sivan SS, et al. Eur Spine J. 2014 Jun;23 Suppl 3:S344-53. doi: 10.1007/s00586-013-2767-8. Epub 2013 Apr 17. Eur Spine J. 2014. PMID: 23591805 Review.
References
- Huxley-Jones J., Apte S. S., Robertson D. L., Boot-Handford R. P. (2005) Int. J. Biochem. Cell Biol. 37, 1838–1845 - PubMed
- Angerer L., Hussain S., Wei Z., Livingston B. T. (2006) Dev. Biol. 300, 267–281 - PubMed
- Le Goff C., Somerville R. P., Kesteloot F., Powell K., Birk D. E., Colige A. C., Apte S. S. (2006) Development 133, 1587–1596 - PubMed
- Levy G. G., Nichols W. C., Lian E. C., Foroud T., McClintick J. N., McGee B. M., Yang A. Y., Siemieniak D. R., Stark K. R., Gruppo R., Sarode R., Shurin S. B., Chandrasekaran V., Stabler S. P., Sabio H., Bouhassira E. E., Upshaw J. D., Jr., Ginsburg D., Tsai H. M. (2001) Nature 413, 488–494 - PubMed
- Zheng X., Chung D., Takayama T. K., Majerus E. M., Sadler J. E., Fujikawa K. (2001) J. Biol. Chem. 276, 41059–41063 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous