Prostate specific antigen for early detection of prostate cancer: longitudinal study - PubMed (original) (raw)
Prostate specific antigen for early detection of prostate cancer: longitudinal study
Benny Holmström et al. BMJ. 2009.
Abstract
Objective: To evaluate if prostate specific antigen test attains validity standards required for screening in view of recent prostate cancer screening trial results.
Design: Case-control study nested in longitudinal cohort.
Setting: Västerbotten Intervention Project cohort, Umeå, Sweden.
Participants: 540 cases and 1034 controls matched for age and date of blood draw.
Main outcome measure: Validity of prostate specific antigen for prediction of subsequent prostate cancer diagnosis by record linkage to cancer registry.
Results: Blood samples were drawn on average 7.1 (SD 3.7) years before diagnosis. The area under the curve for prostate specific antigen was 0.84 (95% confidence interval 0.82 to 0.86). At prostate specific antigen cut-off values of 3, 4, and 5 ng/ml, sensitivity estimates were 59%, 44%, and 33%, and specificity estimates were 87%, 92%, and 95%. The positive likelihood ratio commonly considered to "rule in disease" is 10; in this study the positive likelihood ratios were 4.5, 5.5, and 6.4 for prostate specific antigen cut-off values of 3, 4, and 5 ng/ml. The negative likelihood ratio commonly considered to "rule out disease" is 0.1; in this study the negative likelihood ratios were 0.47, 0.61, and 0.70 for prostate specific antigen cut-off values of 3, 4, and 5 ng/ml. For a cut-off of 1.0 ng/ml, the negative likelihood ratio was 0.08.
Conclusions: No single cut-off value for prostate specific antigen concentration attained likelihood ratios formally required for a screening test. Prostate specific antigen concentrations below 1.0 ng/ml virtually ruled out a prostate cancer diagnosis during the follow-up. Additional biomarkers for early detection of prostate cancer are needed before population based screening for prostate cancer should be introduced.
Conflict of interest statement
Conflicts of interest: None declared.
Figures
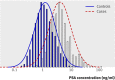
Fig 1 Distribution of plasma prostate specific antigen (PSA) concentrations in cases and controls. Curves indicate frequency functions of calculated normal distribution of logarithm of PSA concentrations according to mean and standard deviation in cases and controls. Histogram shows observed distribution of logarithm of PSA concentrations in cases and controls
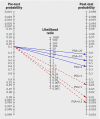
Fig 2 Fagan’s nomogram for calculation of post-test probabilities. Blue (solid) arrows indicate post-test probability of diagnosis during follow-up for men with prostate specific antigen (PSA) concentrations above given cut-offs. Red (broken) arrows indicate post-test probability of diagnosis during follow-up for men with PSA concentrations below given cut-offs
Comment in
- Prostate specific antigen for detecting early prostate cancer.
Ilic D, Green S. Ilic D, et al. BMJ. 2009 Sep 24;339:b3572. doi: 10.1136/bmj.b3572. BMJ. 2009. PMID: 19778970 No abstract available. - Prostate specific antigen. Prostate screening in France.
Braillon A. Braillon A. BMJ. 2009 Oct 21;339:b4285. doi: 10.1136/bmj.b4285. BMJ. 2009. PMID: 19846492 No abstract available. - PSA and prostate cancer. More research is not needed.
Wright CJ. Wright CJ. BMJ. 2009 Dec 10;339:b5336. doi: 10.1136/sbmj.b5336. BMJ. 2009. PMID: 20007232 No abstract available.
Similar articles
- Combining 33 genetic variants with prostate-specific antigen for prediction of prostate cancer: longitudinal study.
Johansson M, Holmström B, Hinchliffe SR, Bergh A, Stenman UH, Hallmans G, Wiklund F, Stattin P. Johansson M, et al. Int J Cancer. 2012 Jan 1;130(1):129-37. doi: 10.1002/ijc.25986. Epub 2011 Apr 1. Int J Cancer. 2012. PMID: 21328341 - Usefulness of prostate-specific antigen velocity in screening for prostate cancer.
Ito K, Yamamoto T, Ohi M, Kubota Y, Fukabori Y, Kurokawa K, Suzuki K, Yamanaka H. Ito K, et al. Int J Urol. 2002 Jun;9(6):316-21. doi: 10.1046/j.1442-2042.2002.00466.x. Int J Urol. 2002. PMID: 12110095 - Comparative study of total prostate specific antigen and free to total prostate specific antigen ratio in the diagnosis of prostate cancer.
Chakraborty L, Ahmed AN, Paul BK, Haque A, Ara A, Nabi S, Nessa M. Chakraborty L, et al. Mymensingh Med J. 2012 Jan;21(1):98-102. Mymensingh Med J. 2012. PMID: 22314462 - Tumor markers. Consensus Conference on Diagnosis and Prognostic Parameters in Localized Prostate Cancer. Stockholm, Sweden, May 12-13, 1993.
Stamey TA, Ekman PE, Blankenstein MA, Cooper EH, Kontturi M, Lilja H, Oesterling JE, Stenman UH, Turkes A. Stamey TA, et al. Scand J Urol Nephrol Suppl. 1994;162:73-87; discussion 115-27. Scand J Urol Nephrol Suppl. 1994. PMID: 7529430 Review. - Prostate specific antigen in the diagnosis and staging of prostate cancer.
Jung P, Wolff JM, Mattelaer P, Jakse G. Jung P, et al. Acta Urol Belg. 1996 Sep;64(3):1-6. Acta Urol Belg. 1996. PMID: 8946774 Review.
Cited by
- The Use of Magnetic Resonance Imaging in the Prostate Cancer Primary Diagnostic Pathway: Is It Ready for Primetime?
Sathianathen NJ, Warlick CA. Sathianathen NJ, et al. World J Mens Health. 2018 Sep;36(3):223-229. doi: 10.5534/wjmh.2018.180025. World J Mens Health. 2018. PMID: 30168298 Free PMC article. Review. - Microfluidic and Paper-Based Devices for Disease Detection and Diagnostic Research.
Campbell JM, Balhoff JB, Landwehr GM, Rahman SM, Vaithiyanathan M, Melvin AT. Campbell JM, et al. Int J Mol Sci. 2018 Sep 12;19(9):2731. doi: 10.3390/ijms19092731. Int J Mol Sci. 2018. PMID: 30213089 Free PMC article. Review. - Urinary aHGF, IGFBP3 and OPN as diagnostic and prognostic biomarkers for prostate cancer.
Prager AJ, Peng CR, Lita E, McNally D, Kaushal A, Sproull M, Compton K, Dahut WL, Figg WD, Citrin D, Camphausen KA. Prager AJ, et al. Biomark Med. 2013 Dec;7(6):831-41. doi: 10.2217/bmm.13.112. Biomark Med. 2013. PMID: 24266816 Free PMC article. - Simulation model of disease incidence driven by diagnostic activity.
Westerberg M, Larsson R, Holmberg L, Stattin P, Garmo H. Westerberg M, et al. Stat Med. 2021 Feb 28;40(5):1172-1188. doi: 10.1002/sim.8833. Epub 2020 Nov 25. Stat Med. 2021. PMID: 33241594 Free PMC article.
References
- Ross KS, Carter HB, Pearson JD, Guess HA. Comparative efficiency of prostate-specific antigen screening strategies for prostate cancer detection. JAMA 2000;284:1399-405. - PubMed
- Yao SL, Lu-Yao G. Interval after prostate specific antigen testing and subsequent risk of incurable prostate cancer. J Urol 2001;166:861-5. - PubMed
- Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2008: a review of current American Cancer Society guidelines and cancer screening issues. CA Cancer J Clin 2008;58:161-79. - PubMed
- Lieberman R. Evidence-based medical perspectives: the evolving role of PSA for early detection, monitoring of treatment response, and as a surrogate end point of efficacy for interventions in men with different clinical risk states for the prevention and progression of prostate cancer. Am J Ther 2004;11:501-6. - PubMed
- Stenman UH, Abrahamsson PA, Aus G, Lilja H, Bangma C, Hamdy FC, et al. Prognostic value of serum markers for prostate cancer. Scand J Urol Nephrol Suppl 2005;216:64-81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical