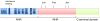NFAT proteins: emerging roles in cancer progression - PubMed (original) (raw)
Review
NFAT proteins: emerging roles in cancer progression
Maria Mancini et al. Nat Rev Cancer. 2009 Nov.
Abstract
The roles of nuclear factor of activated T cells (NFAT) transcription factors have been extensively studied in the immune system. However, ubiquitous expression of NFAT isoforms in mammalian tissues has recently been observed, and a role for these transcription factors in human cancer is emerging. Various NFAT isoforms are functional in tumour cells and multiple compartments in the tumour microenvironment, including fibroblasts, endothelial cells and infiltrating immune cells. How do NFAT isoforms regulate the complex interplay between these compartments during carcinoma progression? The answers lie with the multiple functions attributed to NFATs, including cell growth, survival, invasion and angiogenesis. In addition to elucidating the complex role of NFATs in cancer, we face the challenge of targeting this pathway therapeutically.
Conflict of interest statement
Competing Financial Interests: The authors declare no competing financial interests.
Figures
Figure 1. Primary structure of NFAT
Schematic structure of NFAT. The domains depicted are retained in NFAT isoforms 1–4, but in NFAT5 the NHR region is truncated and lacks the calcineurin-binding site. The structure shows the NFAT-homology region (NHR) region that comprises the amino-terminal transactivation domain (TAD), the calcineurin-binding site, the nuclear localization sequence (NLS) and the serine-rich regions SRR1, SRR2, and SRR3, as well as the SP1 and SP2 motifs (Ser-Pro rich) that are targeted by maintenance and export kinases. The Rel-homology region (RHR) comprises the DNA binding motif and points of contact with transcriptional binding partners such as Fos and Jun. Note that NFAT1-4 differ in the size of the carboxyl-terminal domain, and alternative splice variants of NFAT1 (isoforms A, B, C), NFAT2 (isoforms A, B, C) and NFAT4 (isoforms 1, 2, 3, 4) also exist that differ in the length of the carboxyl-terminus.
Figure 2. Calcium signaling and activation of NFAT
Receptor tyrosine kinases (RTKs) and immunoreceptors such as the T cell receptor (TCR) activate phospholipase Cγ (PLCγ), which hydrolyses phosphatidylinositol-4,5-bisphosphate (PIP2) releasing inositol-1,4,5-triphosphate (InsP3) and diacylglycerol (DG). InsP3 and loss of calcium binding on STIM1 (stromal interaction molecule 1) induces calcium release from the endoplasmic reticulum (ER). Calcium-release-activated calcium channels (CRACs), including Orai1, are then opened, allowing a sustained influx of extracellular calcium. Calmodulin (CAM) binds calcium and in turn the phosphatase calcineurin (CaN). Binding of calcium to the calcineurin regulatory B subunit (CaNB) exposes the calmodulin-binding site on the catalytic A subunit (CaNA). An autoinhibitory sequence in calcineurin is then released from the catalytic pocket, and the phosphatase can dephosphorylate cytoplasmic nuclear factor of activated T cells (NFAT). Inactive NFAT is basally hyperphosphorylated; dephosphorylation promotes nuclear translocation and gene transcription. NFAT cooperates with multiple other transcription factors, including the activator protein 1 (AP-1) complex (Fos-Jun dimers). RTK and TCR activation also stimulates signaling through the MAPK pathway leading to AP-1 activation. The NFAT activation cycle is maintained through complex mechanisms of maintenance kinases that retain cytoplasmic hyperphosphorylated NFAT, such as casein kinase-1 (CK1) and dual-specificity tyrosine-phosphorylation regulated kinase 2 (DYRK2), as well as nuclear export kinases such as CK1, DYRK1 and GSK-3 (glycogen synthase kinase-3). These kinases are counteracted by negative regulators of calcineurin, such as DSCR1 (Down syndrome candidate region 1). Pharmacological antagonists of calcineurin, such as FK506 and cyclosporin A (CsA) are potent inhibitors of NFAT dephosphorylation and nuclear accumulation.
Figure 3. NFAT promotes tumor cell migration through paracrine and autocrine mechanisms
Subsequent to genetic and epigenetic deregulation, epithelial cells that reside on the basement membrane undergo a fundamental change in morphology adopting a mesenchymal architecture, concomitant with acquisition of a motile phenotype. This ordered series of events is collectively termed the epithelial to mesenchymal transition (EMT). Subsequently, the tumor cells degrade the basement membrane and this facilitates cancer invasion into connective tissue that is comprised primarily of extracellular membrane (ECM) proteins. NFAT promotes migration and invasion through multiple non-redundant mechanisms. Engagement of integrins such as α6β4 on tumor cells promotes NFAT nuclear translocation likely through calcium flux. Nuclear NFAT transactivates numerous genes, including those that encode the autotaxin and cyclooxygenase (COX-2) proteins. Autotaxin and COX-2 are secreted proteins and catalyze the synthesis of lysophosphatidic acid (LPA) and prostaglandin E2 (PGE2) respectively. Both LPA and PGE2 are potent motogens and mitogens that act in both paracrine and autocrine signaling through binding Edg (endothelial differentiation gene) and prostaglandin E2 (EP) receptors, respectively, to promote the invasive migration of tumor cells through the ECM.
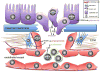
Figure 4. NFAT promotes tumor angiogenesis
The vascular endothelial growth factor (VEGF) is secreted into the tumor microenvironment by multiple distinct resident cell types, including endothelial cells, fibroblasts and the tumor cells themselves. If not immediately utilized, VEGF is tethered to the extracellular matrix (ECM), and signals that stimulate the angiogenic switch during tumor progression activate mesenchymal stem cells (MSCs), secretion of matrix metalloproteases and release VEGF from the ECM. VEGF then binds VEGF-receptors (VEGF-R1 and/or 2), leading to an increase in intracellular calcium that promotes the nuclear translocation of NFAT. Activation of NFAT in endothelial cells induces the transcription of VEGF and VEGF-R that function in an autocrine loop. NFAT also induces COX-2 in endothelial cells leading to synthesis of PGE2, which binds to Edg receptors. Both VEGF and PGE2 stimulate endothelial cell proliferation, migration and ultimately vessel formation. Endogenous inhibitors of calcineurin-NFAT, such as DSCR1 (Down’s syndrome candidate region 1) block endothelial cell NFAT activation and are potent inhibitors of tumor angiogenesis.
Figure 5. Multiple roles for NFAT in the heterotypic interactions of the tumor microenvironment
Multiple non-redundant mechanisms function to control the specific roles of NFAT transcription factors in tumor progression. In non-tumorigenic epithelial cells, quiescence is achieved by multiple mechanisms, including the NFAT-dependent suppression of cyclin-dependent kinases (CDK4) that are required for proliferation. During tumorigenesis, cells acquire a motile and invasive phenotype that is in part dependent on the upregulation of NFAT activity that in turn stimulates the synthesis and secretion of pro-motility factors such as lysophosphatidic acid (LPA) and prostaglandin E2 (PGE2). Intravasation of tumor cells into the vasculature is only possible subsequent to the angiogenic switch. NFAT promotes angiogenesis through secretion of pro-angiogenic factors such as the vascular endothelial growth factor (VEGF) that are secreted by endothelial cells and fibroblasts in an NFAT-dependent manner. Infiltrating immune cells are mobilized to the tumor microenvironment by chemotaxis mechanisms that depend on NFAT activation, and in turn secrete local factors such as colony-stimulating factor-1 (CSF-1) that promote proliferation.
Similar articles
- Nuclear factor of activated T cells in cancer development and treatment.
Shou J, Jing J, Xie J, You L, Jing Z, Yao J, Han W, Pan H. Shou J, et al. Cancer Lett. 2015 Jun 1;361(2):174-84. doi: 10.1016/j.canlet.2015.03.005. Epub 2015 Mar 10. Cancer Lett. 2015. PMID: 25766658 Review. - NFAT as cancer target: mission possible?
Qin JJ, Nag S, Wang W, Zhou J, Zhang WD, Wang H, Zhang R. Qin JJ, et al. Biochim Biophys Acta. 2014 Dec;1846(2):297-311. doi: 10.1016/j.bbcan.2014.07.009. Epub 2014 Jul 26. Biochim Biophys Acta. 2014. PMID: 25072963 Free PMC article. Review. - Differential regulation of calcium-NFAT signaling pathway by Akt isoforms: unraveling effector dynamics and exhaustion of cytotoxic T lymphocytes in tumor microenvironment.
Chen WL, Chang YL, Lin SF, Protzer U, Isogawa M, Yang HC, Huang LR. Chen WL, et al. J Immunother Cancer. 2025 Mar 26;13(3):e009827. doi: 10.1136/jitc-2024-009827. J Immunother Cancer. 2025. PMID: 40139836 Free PMC article. - NFAT signaling dysregulation in cancer: Emerging roles in cancer stem cells.
Lin Y, Song Y, Zhang Y, Shi M, Hou A, Han S. Lin Y, et al. Biomed Pharmacother. 2023 Sep;165:115167. doi: 10.1016/j.biopha.2023.115167. Epub 2023 Jul 14. Biomed Pharmacother. 2023. PMID: 37454598 Review. - NFAT transcription factors: from cell cycle to tumor development.
Viola JP, Carvalho LD, Fonseca BP, Teixeira LK. Viola JP, et al. Braz J Med Biol Res. 2005 Mar;38(3):335-44. doi: 10.1590/s0100-879x2005000300003. Epub 2005 Mar 8. Braz J Med Biol Res. 2005. PMID: 15761612 Review.
Cited by
- Emerging roles of store-operated Ca²⁺ entry through STIM and ORAI proteins in immunity, hemostasis and cancer.
Bergmeier W, Weidinger C, Zee I, Feske S. Bergmeier W, et al. Channels (Austin). 2013 Sep-Oct;7(5):379-91. doi: 10.4161/chan.24302. Epub 2013 Mar 19. Channels (Austin). 2013. PMID: 23511024 Free PMC article. Review. - Cot kinase promotes Ca2+ oscillation/calcineurin-independent osteoclastogenesis by stabilizing NFATc1 protein.
Kuroda Y, Hisatsune C, Mizutani A, Ogawa N, Matsuo K, Mikoshiba K. Kuroda Y, et al. Mol Cell Biol. 2012 Jul;32(14):2954-63. doi: 10.1128/MCB.05611-11. Epub 2012 May 21. Mol Cell Biol. 2012. PMID: 22615493 Free PMC article. - Unraveled roles of Cav1.2 in proliferation and stemness of ameloblastoma.
Li S, Lee DJ, Kim HY, Kim JY, Jung YS, Jung HS. Li S, et al. Cell Biosci. 2022 Sep 3;12(1):145. doi: 10.1186/s13578-022-00873-9. Cell Biosci. 2022. PMID: 36057617 Free PMC article. - Multiscale-omic assessment of EWSR1-NFATc2 fusion positive sarcomas identifies the mTOR pathway as a potential therapeutic target.
Seligson ND, Maradiaga RD, Stets CM, Katzenstein HM, Millis SZ, Rogers A, Hays JL, Chen JL. Seligson ND, et al. NPJ Precis Oncol. 2021 May 21;5(1):43. doi: 10.1038/s41698-021-00177-0. NPJ Precis Oncol. 2021. PMID: 34021224 Free PMC article. - B-cell receptor-mediated NFATc1 activation induces IL-10/STAT3/PD-L1 signaling in diffuse large B-cell lymphoma.
Li L, Zhang J, Chen J, Xu-Monette ZY, Miao Y, Xiao M, Young KH, Wang S, Medeiros LJ, Wang M, Ford RJ, Pham LV. Li L, et al. Blood. 2018 Oct 25;132(17):1805-1817. doi: 10.1182/blood-2018-03-841015. Epub 2018 Sep 12. Blood. 2018. PMID: 30209121 Free PMC article.
References
- Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109 (Suppl):S67–79. - PubMed
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–32. - PubMed
- Shaw JP, et al. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–5. - PubMed
- Jauliac S, et al. The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat Cell Biol. 2002;4:540–4. This is the first study to demonstrate that NFAT is a pro-migration and invasion transcription factor in breast cancer. - PubMed
- Medyouf H, et al. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat Med. 2007;13:736–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases