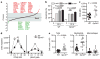Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43 - PubMed (original) (raw)
. 2009 Oct 29;461(7268):1282-6.
doi: 10.1038/nature08530.
Angelica T Vieira, Aylwin Ng, Jan Kranich, Frederic Sierro, Di Yu, Heidi C Schilter, Michael S Rolph, Fabienne Mackay, David Artis, Ramnik J Xavier, Mauro M Teixeira, Charles R Mackay
Affiliations
- PMID: 19865172
- PMCID: PMC3256734
- DOI: 10.1038/nature08530
Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43
Kendle M Maslowski et al. Nature. 2009.
Abstract
The immune system responds to pathogens by a variety of pattern recognition molecules such as the Toll-like receptors (TLRs), which promote recognition of dangerous foreign pathogens. However, recent evidence indicates that normal intestinal microbiota might also positively influence immune responses, and protect against the development of inflammatory diseases. One of these elements may be short-chain fatty acids (SCFAs), which are produced by fermentation of dietary fibre by intestinal microbiota. A feature of human ulcerative colitis and other colitic diseases is a change in 'healthy' microbiota such as Bifidobacterium and Bacteriodes, and a concurrent reduction in SCFAs. Moreover, increased intake of fermentable dietary fibre, or SCFAs, seems to be clinically beneficial in the treatment of colitis. SCFAs bind the G-protein-coupled receptor 43 (GPR43, also known as FFAR2), and here we show that SCFA-GPR43 interactions profoundly affect inflammatory responses. Stimulation of GPR43 by SCFAs was necessary for the normal resolution of certain inflammatory responses, because GPR43-deficient (Gpr43(-/-)) mice showed exacerbated or unresolving inflammation in models of colitis, arthritis and asthma. This seemed to relate to increased production of inflammatory mediators by Gpr43(-/-) immune cells, and increased immune cell recruitment. Germ-free mice, which are devoid of bacteria and express little or no SCFAs, showed a similar dysregulation of certain inflammatory responses. GPR43 binding of SCFAs potentially provides a molecular link between diet, gastrointestinal bacterial metabolism, and immune and inflammatory responses.
Figures
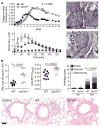
Figure 1. Exacerbated colitis in germ-free mice is ameliorated by acetate
a, Germ-free (open squares) and CNV (closed triangles) mice were given DSS colitis (4%), n = 7 (experimental groups). Dashed lines, control mice; solid lines, DSS-treated mice. The percentage weight change (left), DAI (middle) and haematocrit (right) were measured. b, Germ-free mice were fed acetate (grey squares; 150 mM; n = 3) in the drinking water or water only (black squares; n = 5), 5 days before and during DSS administration. Control fed denotes no DSS (open squares). Daily activity score (left), colon length (middle) and colonic MPO (right) were determined. c, MIP1α and TNFα levels in acetate-fed mice. Data are median ± s.e.m., representative of two independent experiments.
Figure 2. GPR43 expression and role in inflammatory responses
a, Immune expression signature of genes encoding cellular receptors across a large panel of leukocyte subsets. Clustering of receptor genes exhibiting enriched expression in neutrophils and eosinophils reveals GPR43, along with other receptors important for innate immunity and chemoattractant-induced responses. Correlation analysis across a wider set of genes in this immune panel identified a rank-ordered list of the top 150 genes (_N_150) in the co-expression neighbourhood of GPR43. b–e, Comparison of wild-type (WT) and _Gpr43_−/− bone marrow neutrophils with respect to acetate-induced Ca2+ flux (b; MFI, mean fluorescence intensity), chemotaxis (c, left panel), ROS production (d), and phagocytosis of fluorescently labelled S. aureus (e). The right panel of c shows the GPR43 synthetic agonist phenylacetamide 1 in human neutrophil chemotaxis. f, DSS colitis (2.5% (w/v)) in wild-type and _Gpr43_−/− mice, fed with acetate or control water (Ctrl). Shown are colon length, the DAI, histology score and colon MPO levels. NS, not significant. The far right panels show representative histological sections from wild-type or _Gpr43_−/− mice as indicated (scale bar, 50 μm). g, Chronic DSS-induced colitis (n = 7 per group, median ± s.e.m.). The inset shows the percentage morbidity. Shown are the percentage change in weight, colon weight per cm of colon, colonic MPO, and histological score, for wild-type and _Gpr43_−/− mice.
Figure 3. Inflammatory arthritis and allergic airway disease and GPR43 deficiency
a, Inflammatory arthritis (K/BxN serum injection on day 0 and 2) in _Gpr43_−/− mice (n = 5) versus wild-type littermates (n ≥ 3). Scores shown are mean ± s.e.m. for each time point, representative of three independent experiments. Wild-type mice are represented with closed squares, _Gpr43_−/− mice with open squares, controls with dashed lines, and arthritic mice with solid lines. Change in ankle thickness (top) and measurement of MPO in the peripheral blood (bottom) showed that both naive and arthritic _Gpr43_−/− mice had higher MPO production when stimulated with phorbol 12-myristate 13-acetate (PMA; bottom), indicating greater neutrophil activation (P < 0.001 _Gpr43_−/− control compared to wild-type control, P = 0.0019 _Gpr43_−/− compared to wild-type arthritic). Histological assessment at day 18 (right) (scale bars, 50 μm). b, OVA-induced allergic airway inflammation. BAL fluid cell counts (left), eosinophil peroxidase (EPO) activity in lung tissue (middle), and inflammation as scored by histology (right). The bottom panel shows representative haematoxylin-and-eosin-stained lung sections from wild-type and _Gpr43_−/− mice, and control (no OVA) mice. Scale bar, 50 μm.
Figure 4. GPR43 signalling and immune cell functions
a, Protein expression analysis using Kinex antibody microarrays. _Z_-score-transformed values reflecting positive or negative shifts in differential protein expression fold-changes after acetate treatment of neutrophils from wild-type mice compared to that from _Gpr43_−/− mice. Proteins highlighted in red or green indicate those with _Z_-scores above +1.5 or below −1.5, respectively. b, Apoptosis in wild-type and _Gpr43_−/− bone marrow cells, with or without acetate stimulation (apoptotic cells are annexin V and propidium iodide (PI) double positive). c, Chemotactic response to fMLP and C5a by wild-type and _Gpr43_−/− bone marrow granulocytes. d, Recruitment of neutrophils and macrophages to the peritoneum in wild-type and _Gpr43_−/− mice, injected with 1 × 106 heat-inactivated S. aureus particles. e, Reactive oxygen species production by peripheral blood granulocytes.
Comment in
- Metabolic bridge between microbiota and humans.
Takeda K. Takeda K. Nat Rev Immunol. 2016 Apr;16(4):206. doi: 10.1038/nri.2016.18. Nat Rev Immunol. 2016. PMID: 26852927 No abstract available.
Similar articles
- Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice.
Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Kim MH, et al. Gastroenterology. 2013 Aug;145(2):396-406.e1-10. doi: 10.1053/j.gastro.2013.04.056. Epub 2013 May 7. Gastroenterology. 2013. PMID: 23665276 - G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells.
Masui R, Sasaki M, Funaki Y, Ogasawara N, Mizuno M, Iida A, Izawa S, Kondo Y, Ito Y, Tamura Y, Yanamoto K, Noda H, Tanabe A, Okaniwa N, Yamaguchi Y, Iwamoto T, Kasugai K. Masui R, et al. Inflamm Bowel Dis. 2013 Dec;19(13):2848-56. doi: 10.1097/01.MIB.0000435444.14860.ea. Inflamm Bowel Dis. 2013. PMID: 24141712 - Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome.
Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. Macia L, et al. Nat Commun. 2015 Apr 1;6:6734. doi: 10.1038/ncomms7734. Nat Commun. 2015. PMID: 25828455 - GPR43 - A Prototypic Metabolite Sensor Linking Metabolic and Inflammatory Diseases.
McKenzie CI, Mackay CR, Macia L. McKenzie CI, et al. Trends Endocrinol Metab. 2015 Oct;26(10):511-512. doi: 10.1016/j.tem.2015.07.009. Trends Endocrinol Metab. 2015. PMID: 26412151 Review. - [Host energy regulation via SCFAs receptors, as dietary nutrition sensors, by gut microbiota].
Kimura I. Kimura I. Yakugaku Zasshi. 2014;134(10):1037-42. doi: 10.1248/yakushi.14-00169. Yakugaku Zasshi. 2014. PMID: 25274213 Review. Japanese.
Cited by
- Sodium butyrate protects against lipopolysaccharide-induced liver injury partially via the GPR43/ β-arrestin-2/NF-κB network.
Luo QJ, Sun MX, Guo YW, Tan SW, Wu XY, Abassa KK, Lin L, Liu HL, Jiang J, Wei XQ. Luo QJ, et al. Gastroenterol Rep (Oxf). 2020 Nov 22;9(2):154-165. doi: 10.1093/gastro/goaa085. eCollection 2021 Apr. Gastroenterol Rep (Oxf). 2020. PMID: 34026223 Free PMC article. - Causal Effects of Gut Microbiota and Metabolites on Chronic Obstructive Pulmonary Disease: A Bidirectional Two Sample Mendelian Randomization Study.
Du Y, Wang S, Zhou T, Zhao Z. Du Y, et al. Int J Chron Obstruct Pulmon Dis. 2024 Sep 28;19:2153-2167. doi: 10.2147/COPD.S472218. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 39360021 Free PMC article. - Influence of gut microbiota on subclinical inflammation and insulin resistance.
Carvalho BM, Saad MJ. Carvalho BM, et al. Mediators Inflamm. 2013;2013:986734. doi: 10.1155/2013/986734. Epub 2013 Jun 12. Mediators Inflamm. 2013. PMID: 23840101 Free PMC article. Review. - The impact of dietary fibers on Clostridioides difficile infection in a mouse model.
Wu Z, Xu Q, Wang Q, Chen Y, Lv L, Zheng B, Yan R, Jiang H, Shen J, Wang S, Wang K, Xia J, Han S, Li L. Wu Z, et al. Front Cell Infect Microbiol. 2022 Nov 9;12:1028267. doi: 10.3389/fcimb.2022.1028267. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36439215 Free PMC article. - Short Chain Fatty Acids: Essential Weapons of Traditional Medicine in Treating Inflammatory Bowel Disease.
Yao Y, Liu Y, Xu Q, Mao L. Yao Y, et al. Molecules. 2024 Jan 12;29(2):379. doi: 10.3390/molecules29020379. Molecules. 2024. PMID: 38257292 Free PMC article. Review.
References
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. - PubMed
- Treem WR, Ahsan N, Shoup M, Hyams JS. Fecal short-chain fatty acids in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1994;18:159–164. - PubMed
- Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320:23–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI074878/AI/NIAID NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R01 AI095466/AI/NIAID NIH HHS/United States
- R01 AI061570-06/AI/NIAID NIH HHS/United States
- P30 DK040561-14/DK/NIDDK NIH HHS/United States
- R01 HL088297/HL/NHLBI NIH HHS/United States
- R01 AI074878-02/AI/NIAID NIH HHS/United States
- R01 HL088297-02/HL/NHLBI NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases