Phosphorylation of synucleins by members of the Polo-like kinase family - PubMed (original) (raw)
. 2010 Jan 22;285(4):2807-22.
doi: 10.1074/jbc.M109.081950. Epub 2009 Nov 4.
Katerina E Paleologou, Ahmed Boucharaba, Abid Oueslati, Heinrich Schell, Margot Fournier, Diana Olschewski, Guowei Yin, Markus Zweckstetter, Eliezer Masliah, Philipp J Kahle, Harald Hirling, Hilal A Lashuel
Affiliations
- PMID: 19889641
- PMCID: PMC2807335
- DOI: 10.1074/jbc.M109.081950
Phosphorylation of synucleins by members of the Polo-like kinase family
Martial K Mbefo et al. J Biol Chem. 2010.
Abstract
Phosphorylation of alpha-synuclein (alpha-syn) at Ser-129 is a hallmark of Parkinson disease and related synucleinopathies. However, the identity of the natural kinases and phosphatases responsible for regulating alpha-syn phosphorylation remain unknown. Here we demonstrate that three closely related members of the human Polo-like kinase (PLK) family (PLK1, PLK2, and PLK3) phosphorylate alpha-syn and beta-syn specifically at Ser-129 and Ser-118, respectively. Unlike other kinases reported to partially phosphorylate alpha-syn at Ser-129 in vitro, phosphorylation by PLK2 and PLK3 is quantitative (>95% conversion). Only PLK1 and PLK3 phosphorylate beta-syn at Ser-118, whereas no phosphorylation of gamma-syn was detected by any of the four PLKs (PLK1 to -4). PLK-mediated phosphorylation was greatly reduced in an isolated C-terminal fragment (residues 103-140) of alpha-syn, suggesting substrate recognition via the N-terminal repeats and/or the non-amyloid component domain of alpha-syn. PLKs specifically co-localized with phosphorylated Ser-129 (Ser(P)-129) alpha-syn in various subcellular compartments (cytoplasm, nucleus, and membranes) of mammalian cell lines and primary neurons as well as in alpha-syn transgenic mice, especially cortical brain areas involved in synaptic plasticity. Furthermore, we report that the levels of PLK2 are significantly increased in brains of Alzheimer disease and Lewy body disease patients. Taken together, these results provide biochemical and in vivo evidence of alpha-syn and beta-syn phosphorylation by specific PLKs. Our results suggest a need for further studies to elucidate the potential role of PLK-syn interactions in the normal biology of these proteins as well as their involvement in the pathogenesis of Parkinson disease and other synucleinopathies.
Figures
FIGURE 1.
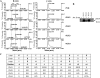
Schematic depiction of the sequences of synucleins and PLK1s-4. A, schematic depictions illustrating the sequence similarities and differences among the four PLKs and members of the synuclein family of proteins. The four members of the PLK family share a conserved N-terminal kinase domain. The PBD of PLK1, PLK2, and PLK3 contain two tandem Polo boxes (∼80 residues in length) that associate to form a phosphopeptide binding site, whereas PLK4 possesses only a single PB, which mediates the dimerization of PLK4. B, sequence alignment of the human WT α-, β-, and γ-syn generated by the Clustal 2.0.8 multiple sequence alignment program. Ser-129 in α-syn and Ser-118 are highlighted within the square. aa, amino acids.
FIGURE 2.
In vitro phosphorylation of synucleins by PLK1 to -4. A, MALDI-TOF analysis of the WT α-syn after phosphorylation by PLK1 to -4. For PLK1 to -3, there is an 80-Da increase in the molecular mass of WT α-syn (14,461 + 80 = 14,541), corresponding to one phosphorylation. B, Western blot analysis of the same samples in A. The anti-Ser(P)-129 antibody detected a band, suggesting that the phosphorylation detected by mass spectrometry is at position 129. C, comparison of two-dimensional 1H,15N HSQC spectra of unphosphorylated WT (green) and α-syn phosphorylated by PLK3 (red). A dashed rectangle marks glutamine (Q) and asparagine (N) side chain resonances. D, kinetics of in vitro phosphorylation of Ser-129 in α-syn by PLK3 (black), PLK2 (red), and PLK1 (blue) as monitored by real-time NMR spectroscopy. NMR samples contained ∼0.1 m
m
15N-labeled α-syn in 200 m
m
HEPES, 10 m
m
MgCl2, 2 m
m
dithiothreitol, and 1.09 m
m
ATP, pH 6.9. The real-time assay was started by the addition of kinase into the NMR sample using a protein/kinase ratio of 100:0.5 mg. The error bars were determined based on the signal/noise ratio observed in the NMR spectra.
FIGURE 3.
Recombinant β-syn is phosphorylated by PLK1 and PLK3, whereas γ-syn does not undergo phosphorylation by PLK1 to -4. A, MALDI-TOF analysis of the in vitro phosphorylation reaction of WT β- and γ-syn by PLK1 to -4. WT β-syn undergoes phosphorylation by PLK1 and PLK3 but not PLK2 and PLK4. None of the recombinant PLKs were observed to phosphorylate γ-syn in vitro. B, Western blot analysis of β-syn samples phosphorylated by PLK1 to -4, using our anti-phosphoserine 118 (pS118) antibody. The table in C shows the consensus substrate specificity of PLK1 to -4 (ψ, hydrophobic amino acid) and the amino acids adjacent to the Ser-129 in α-syn and its equivalent residues in β- and γ-syn.
FIGURE 4.
Removal of the entire N-terminal residues (positions 1–103) abolishes Ser-129 phosphorylation, whereas deletion of the NAC residues 73–83 has no effect on Ser-129 phosphorylation by PLK1 to -3. A, MALDI-TOF analysis of the PLK-phosphorylated Δ1–103 α-syn, Δ73–83 α-syn, and chimeric β-syn(χ1) proteins. B, schematic representation of the WT α- and β-syn, deletion α-syn Δ73–83 and chimeric β-syn(χ1) proteins. C, Western blotting analysis of the in vitro phosphorylation of these constructs at Ser-129 by PLK1 to -4 using anti Ser(P)-129 antibody (1:5000; Wako). D, Western blotting analysis of the PLK-phosphorylated WT and chimeric β-syn(χ1) probed with Ser(P)-118 antibody (1:250) (1000 ng/lane). a.a., amino acids.
FIGURE 5.
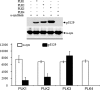
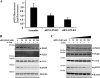
In vitro phosphorylation of α-syn fibrils by PLK1 to -4. Top, preformed α- fibrils were incubated with recombinant PLKs in the appropriate reaction buffers, and phosphorylation at Ser-129 was assessed by anti-Ser(P)-129 antibodies. Bottom, quantification of the level of Ser(P)-129 signal normalized against α-syn and β-actin (pS118/(α-syn + β actin)), n = 3.
FIGURE 6.
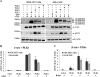
Phosphorylation of α-syn and β-syn by PLKs in mammalian cells (HEK-293T and HeLa). A, the cells were co-transfected with pAAV-CMV-α-syn-WT and different pCMV6-Èntry-PLKs and lysed 24 h post-transfection. The expression level of PLKs and α-syn phosphorylation at Ser-129 were detected by immunoblot using antibodies against FLAG tag and anti-Ser(P)-129. B, quantification of the level of Ser(P)-129 signal intensity was normalized against total fluorescence signal of α-syn WT and β-actin (Ser(P)-129/(α-syn + β-actin), n = 3. C, expression of β-syn-WT and Ser(P)-118 in HEK 293T and HeLa cells after co-transfection as previously described was detected by Western blot (data not shown), and quantification of the level of β-syn Ser(P)-118 signal intensity was normalized against the total fluorescence signal of β-syn-WT and β-actin (pS118/(β-syn + β-actin), n = 3.
FIGURE 7.
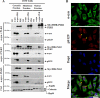
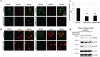
Subcellular localization of α-syn and PLKs in mammalian cell. A, HEK 293T cells were co-transfected with pAAV-CMV-α-syn-WT and different pCMV6-Èntry-PLKs and then subjected to subcellular fractionation 24–48 h post-transfection with the ProteoExtract® subcellular proteome extraction kit from Calbiochem. Purified fractions were then immunoblotted with corresponding antibodies. PARP1, Hsp90, and calnexin proteins were used as control for the nuclear, cytosolic, and membrane particulate fractions, respectively. B, confocal microscopy of HeLa cells co-stained with anti-Ser(P)-129 and anti-α-syn antibodies. Endogenous Ser(P)-129 (red) accumulate exclusively in the nucleus stained with 4′-6′-diamidino-2-phenylindole (Dapi), whereas α-syn WT (green) is mostly membrane and cytosolic in non-transfected cells (NT).
FIGURE 8.
Silencing of PLK2 and PLK3 results in significant reduction of Ser(P)-129 levels in HeLa cell. A, detection of nuclear Ser(P)-129 in non-transfected cells and cells transfected with 100 n
m
ON-TARGET siRNA PLK2 and 100 n
m
ON-TARGET siRNA PLK3. Quantitative analysis of total Ser(P)-129 fluorescence of individual cells was normalized against 4′,6-diamidino-2-phenylindole (Dapi) intensity, n = 3. B and C, immunoblot analysis of crude lysate from HeLa cells sequentially transfected with α-syn and siRNA PLK2 and siRNA PLK3 at the indicated concentration. Anti-β-actin was used as a control of the total amount of proteins loaded.
FIGURE 9.
Silencing of PLK2 and PLK3 significantly inhibit α-syn phosphorylation at Ser-129 in primary neurons. Shown is colocalization (first panel) of Ser(P)-129 and PLKs (A) or WT α-syn versus PLKs (B). C, Western blot analysis of total cell homogenate from 72-h silencing of PLKs in hippocampal neurons. D, quantification of Ser(P)-129 fluorescence signal relative to control (from non-transfected cells). Data are expressed as the mean ± S.D; bar, 100 μ
m
; n = 3.
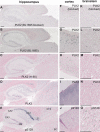
FIGURE 10.
Paraffin-embedded brain serial sections from 1-year-old (Thy1)-[A30P]-αSYN transgenic mice were immunostained with BL1695 anti-PLK2 with (A, F, and K) or without (B, G, and L) preincubation with neutralizing PLK2 protein, H-90 anti-SNK/PLK2 (C, H, and M) anti-PLK3 (D, I, and N), and anti-Ser(P)-129 (E, J, and O). Counterstaining was performed with Nuclear Fast Red. Pictures were taken from the hippocampal region (A–E), neocortex (F–J), and brain stem (K–O). Size bars, 200 μm.
FIGURE 11.
PLK2 expression in diseased brains (AD and LBD). A and B, immunohistochemical analysis of levels of PLK2 in control, AD, and LBD cases. C and D, immunoblot analyses with antibodies against cytosolic α-syn, PLK2, Ser(P)-129, and actin was performed using temporal cortex homogenates from non-demented controls, AD, and LBD cases. Total cellular protein concentrations in each sample were confirmed by comparison with levels of actin (bottom). Bar graph, quantitative image analysis of both immunocytochemical and immunoblotting results are shown.
Similar articles
- In vivo modulation of polo-like kinases supports a key role for PLK2 in Ser129 α-synuclein phosphorylation in mouse brain.
Bergeron M, Motter R, Tanaka P, Fauss D, Babcock M, Chiou SS, Nelson S, San Pablo F, Anderson JP. Bergeron M, et al. Neuroscience. 2014 Jan 3;256:72-82. doi: 10.1016/j.neuroscience.2013.09.061. Epub 2013 Oct 12. Neuroscience. 2014. PMID: 24128992 - Effects of Serine 129 Phosphorylation on α-Synuclein Aggregation, Membrane Association, and Internalization.
Samuel F, Flavin WP, Iqbal S, Pacelli C, Sri Renganathan SD, Trudeau LE, Campbell EM, Fraser PE, Tandon A. Samuel F, et al. J Biol Chem. 2016 Feb 26;291(9):4374-85. doi: 10.1074/jbc.M115.705095. Epub 2015 Dec 30. J Biol Chem. 2016. PMID: 26719332 Free PMC article. - Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions.
Paleologou KE, Oueslati A, Shakked G, Rospigliosi CC, Kim HY, Lamberto GR, Fernandez CO, Schmid A, Chegini F, Gai WP, Chiappe D, Moniatte M, Schneider BL, Aebischer P, Eliezer D, Zweckstetter M, Masliah E, Lashuel HA. Paleologou KE, et al. J Neurosci. 2010 Mar 3;30(9):3184-98. doi: 10.1523/JNEUROSCI.5922-09.2010. J Neurosci. 2010. PMID: 20203178 Free PMC article. - α-Synuclein phosphorylation as a therapeutic target in Parkinson's disease.
Braithwaite SP, Stock JB, Mouradian MM. Braithwaite SP, et al. Rev Neurosci. 2012 Mar 21;23(2):191-8. doi: 10.1515/revneuro-2011-0067. Rev Neurosci. 2012. PMID: 22499677 Review. - Neurotoxic conversion of beta-synuclein: a novel approach to generate a transgenic mouse model of synucleinopathies?
Fujita M, Sekigawa A, Sekiyama K, Sugama S, Hashimoto M. Fujita M, et al. J Neurol. 2009 Aug;256 Suppl 3:286-92. doi: 10.1007/s00415-009-5246-8. J Neurol. 2009. PMID: 19711118 Review.
Cited by
- The Interplay between Alpha-Synuclein Clearance and Spreading.
Lopes da Fonseca T, Villar-Piqué A, Outeiro TF. Lopes da Fonseca T, et al. Biomolecules. 2015 Apr 14;5(2):435-71. doi: 10.3390/biom5020435. Biomolecules. 2015. PMID: 25874605 Free PMC article. Review. - Neurons and Glia Interplay in α-Synucleinopathies.
Mavroeidi P, Xilouri M. Mavroeidi P, et al. Int J Mol Sci. 2021 May 8;22(9):4994. doi: 10.3390/ijms22094994. Int J Mol Sci. 2021. PMID: 34066733 Free PMC article. Review. - Cardiac sympathetic innervation in the MPTP non-human primate model of Parkinson disease.
Carmona-Abellan M, Martínez-Valbuena I, DiCaudo C, Marcilla I, Luquin MR. Carmona-Abellan M, et al. Clin Auton Res. 2019 Aug;29(4):415-425. doi: 10.1007/s10286-019-00620-0. Epub 2019 Jul 23. Clin Auton Res. 2019. PMID: 31338635 - The benefits of humanized yeast models to study Parkinson's disease.
Franssens V, Bynens T, Van den Brande J, Vandermeeren K, Verduyckt M, Winderickx J. Franssens V, et al. Oxid Med Cell Longev. 2013;2013:760629. doi: 10.1155/2013/760629. Epub 2013 Jul 1. Oxid Med Cell Longev. 2013. PMID: 23936613 Free PMC article. Review. - Inhibition of Polo-like kinase 2 ameliorates pathogenesis in Alzheimer's disease model mice.
Lee JS, Lee Y, André EA, Lee KJ, Nguyen T, Feng Y, Jia N, Harris BT, Burns MP, Pak DTS. Lee JS, et al. PLoS One. 2019 Jul 15;14(7):e0219691. doi: 10.1371/journal.pone.0219691. eCollection 2019. PLoS One. 2019. PMID: 31306446 Free PMC article.
References
- Fujiwara H., Hasegawa M., Dohmae N., Kawashima A., Masliah E., Goldberg M. S., Shen J., Takio K., Iwatsubo T. (2002) Nat. Cell Biol. 4, 160–164 - PubMed
- Anderson J. P., Walker D. E., Goldstein J. M., de Laat R., Banducci K., Caccavello R. J., Barbour R., Huang J., Kling K., Lee M., Diep L., Keim P. S., Shen X., Chataway T., Schlossmacher M. G., Seubert P., Schenk D., Sinha S., Gai W. P., Chilcote T. J. (2006) J. Biol. Chem. 281, 29739–29752 - PubMed
- Chen L., Feany M. B. (2005) Nat. Neurosci. 8, 657–663 - PubMed
- Kahle P. J., Neumann M., Ozmen L., Haass C. (2000) Ann. N.Y. Acad. Sci. 920, 33–41 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous