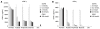Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines - PubMed (original) (raw)
. 2009 Nov 28;15(44):5549-57.
doi: 10.3748/wjg.15.5549.
James Jackson, Michaela Stanton, Alberto Rojas-Triana, Loretta Bober, Maureen Laverty, Xiaoxin Yang, Feng Zhu, Jianjun Liu, Suke Wang, Frederick Monsma, Galya Vassileva, Maureen Maguire, Eric Gustafson, Marvin Bayne, Chuan-Chu Chou, Daniel Lundell, Chung-Her Jenh
Affiliations
- PMID: 19938193
- PMCID: PMC2785057
- DOI: 10.3748/wjg.15.5549
Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines
Mary Ann Cox et al. World J Gastroenterol. 2009.
Abstract
Aim: To investigate the effect of short-chain fatty acids (SCFAs) on production of prostaglandin E(2) (PGE(2)), cytokines and chemokines in human monocytes.
Methods: Human neutrophils and monocytes were isolated from human whole blood by using 1-Step Polymorph and RosetteSep Human Monocyte Enrichment Cocktail, respectively. Human GPR41 and GPR43 mRNA expression was examined by quantitative real-time polymerase chain reaction. The calcium flux assay was used to examine the biological activities of SCFAs in human neutrophils and monocytes. The effect of SCFAs on human monocytes and peripheral blood mononuclear cells (PBMC) was studied by measuring PGE(2), cytokines and chemokines in the supernatant. The effect of SCFAs in vivo was examined by intraplantar injection into rat paws.
Results: Human GPR43 is highly expressed in human neutrophils and monocytes. SCFAs induce robust calcium flux in human neutrophils, but not in human monocytes. In this study, we show that SCFAs can induce human monocyte release of PGE(2) and that this effect can be enhanced in the presence of lipopolysaccharide (LPS). In addition, we demonstrate that PGE(2) production induced by SCFA was inhibited by pertussis toxin, suggesting the involvement of a receptor-mediated mechanism. Furthermore, SCFAs can specifically inhibit constitutive monocyte chemotactic protein-1 (MCP-1) production and LPS-induced interleukin-10 (IL-10) production in human monocytes without affecting the secretion of other cytokines and chemokines examined. Similar activities were observed in human PBMC for the release of PGE(2), MCP-1 and IL-10 after SCFA treatment. In addition, SCFAs inhibit LPS-induced production of tumor necrosis factor-alpha and interferon-gamma in human PBMC. Finally, we show that SCFAs and LPS can induce PGE(2) production in vivo by intraplantar injection into rat paws (P < 0.01).
Conclusion: SCFAs can have distinct antiinflammatory activities due to their regulation of PGE(2), cytokine and chemokine release from human immune cells.
Figures
Figure 1
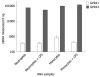
GPR43 is highly expressed in human neutrophils and monocytes. Human neutrophils and monocytes were isolated from human whole blood as described in Materials and Methods. Isolated human neutrophils or monocytes were stimulated with 100 ng/mL of lipopolysaccharide (LPS) for 3 h. RNAs were isolated and evaluated by Taqman analysis for absolute quantities of GPR43 and GPR41 mRNA molecules.
Figure 2
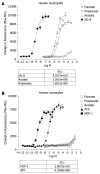
Short-chain fatty acids (SCFAs) induce robust calcium flux in human neutrophils, but not in human monocytes. Human neutrophils (A) and monocytes (B) were isolated and exposed to the indicated concentrations of formate, acetate and propionate for a calcium flux assay as described in Materials and Methods. Interleukin-8 (IL-8) is included as a positive control for human neutrophils, and monocyte chemotactic protein-1 (MCP-1) and ATP for human monocytes. The dose-response curves and EC50 values were obtained by nonlinear regression using Graph Pad Prism 4 software.
Figure 3
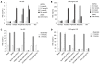
SCFAs strongly induce PGE2 production in human monocytes and the effect is receptor-mediated. Freshly isolated human monocytes (1 × 109 cells/L) were incubated with the indicated concentrations of SCFAs or 100 nmol/L dexamethasone (Dex), and then cultured for 22 h without (A) or with (B) 100 ng/mL LPS. In a separate experiment (C, D), human monocytes were incubated with the indicated concentrations (in the parentheses) of inhibitors (described below) for 30 min, then with or without 2 mmol/L butyrate for another 30 min, and finally cultured for 22 h without (C) or with (D) 100 ng/mL LPS. The media and DMSO samples were used as controls with no inhibitors. All culture supernatants were assayed for PGE2 by ELISA. MLnFP (methyl α-linolenyl fluorophosphonate): Phospholipase inhibitor; 1400W: Selective iNOS inhibitor; SC-560: Selective cyclooxygenase-1 (COX-1) inhibitor; CAY10404: Selective COX-2 inhibitor; PTX: Pertussis toxin. (Cayman Chemical, Ann Arbor, MI). The tested concentrations of each inhibitor (except PTX) were chosen to be about 100 times its IC50 as reported in the data sheet provided by the manufacturer.
Figure 4
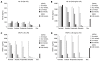
SCFAs specifically inhibit LPS-induced IL-10 production and constitutive MCP-1 expression in human monocytes. The culture supernatants as in Figures 3A and B were analyzed for 8 proinflammatory cytokines (including IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p70, IFN-γ, TNF-α) or 9 proinflammatory chemokines (including MCP-1, MCP-4, eotaxin, eotaxin-3, IL-8, IFN-γ inducible protein-10, macrophage inflammatory protein-1β, macrophage-derived chemokine, thymus and activation-regulated chemokine) by Meso Scale Multi-Spot Discovery Technology. Only the data for IL-10 (A, B) and MCP-1 (C, D) are shown here. Similar results were obtained from 10 human donors.
Figure 5
SCFAs inhibit LPS-induced production of TNF-α and IFN-γ in human peripheral blood mononuclear cells (PBMC) in a dose-dependent manner. Human PBMC isolated by Ficoll-Hypaque centrifugation procedure (1 × 106 cells/mL) were incubated with the indicated concentrations of short-chain fatty acids (SCFA) or 100 nmol/L dexamethasone (Dex), and then cultured for 22 h with or without 100 ng/mL LPS. Culture supernatants were analyzed for 8 proinflammatory cytokines including IL-1β, IL-2, IL-6, IL-8, IL-10, IL-12p70, IFN-γ, TNF-α by Meso Scale Multi-Spot Discovery Technology. The data for TNF-α (A) and IFN-γ (B) with LPS stimulation are shown here.
Figure 6
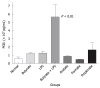
SCFAs induce PGE2 production by intraplantar injection into rat paws. The paw of male Sprague-Dawley rats was injected intraplantarly with 0.1 mL of 100 mmol/L formate, acetate, propionate or butyrate. Three micrograms of LPS in saline was also injected alone or in combination with 0.1 mL of 200 mmol/L butyrate. There were 4 rats per group. The normal group was not injected. Three hours post injection, rat paws were punched and fluids were collected in phenylmethanesulphonyl fluoride buffer with indomethacin. All punch fluids were assayed for PGE2 by ELISA. P < 0.01 for butyrate + LPS group vs normal group, Mann-Whitney U test.
Similar articles
- Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor.
Takayama K, García-Cardena G, Sukhova GK, Comander J, Gimbrone MA Jr, Libby P. Takayama K, et al. J Biol Chem. 2002 Nov 15;277(46):44147-54. doi: 10.1074/jbc.M204810200. Epub 2002 Sep 4. J Biol Chem. 2002. PMID: 12215436 - Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice.
Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Kim MH, et al. Gastroenterology. 2013 Aug;145(2):396-406.e1-10. doi: 10.1053/j.gastro.2013.04.056. Epub 2013 May 7. Gastroenterology. 2013. PMID: 23665276 - Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells.
Kobayashi M, Mikami D, Kimura H, Kamiyama K, Morikawa Y, Yokoi S, Kasuno K, Takahashi N, Taniguchi T, Iwano M. Kobayashi M, et al. Biochem Biophys Res Commun. 2017 Apr 29;486(2):499-505. doi: 10.1016/j.bbrc.2017.03.071. Epub 2017 Mar 18. Biochem Biophys Res Commun. 2017. PMID: 28322790 - Regulation of inflammation by short chain fatty acids.
Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Vinolo MA, et al. Nutrients. 2011 Oct;3(10):858-76. doi: 10.3390/nu3100858. Epub 2011 Oct 14. Nutrients. 2011. PMID: 22254083 Free PMC article. Review. - Systemic inflammatory response to exhaustive exercise. Cytokine kinetics.
Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. Suzuki K, et al. Exerc Immunol Rev. 2002;8:6-48. Exerc Immunol Rev. 2002. PMID: 12690937 Review.
Cited by
- A Sustained-Release Butyrate Tablet Suppresses Ex Vivo T Helper Cell Activation of Osteoarthritis Patients in a Double-Blind Placebo-Controlled Randomized Trial.
Korsten SGPJ, Hartog M, Berends AJ, Koenders MI, Popa CD, Vromans H, Garssen J, van de Ende CHM, Vermeiden JPW, Willemsen LEM. Korsten SGPJ, et al. Nutrients. 2024 Oct 4;16(19):3384. doi: 10.3390/nu16193384. Nutrients. 2024. PMID: 39408351 Free PMC article. Clinical Trial. - Supplementation with Citrus Low-Methoxy Pectin Reduces Levels of Inflammation and Anxiety in Healthy Volunteers: A Pilot Controlled Dietary Intervention Study.
Vijay A, Kelly A, Miller S, Marshall M, Alonso A, Kouraki A, Probert C, Simpson EJ, Valdes AM. Vijay A, et al. Nutrients. 2024 Sep 30;16(19):3326. doi: 10.3390/nu16193326. Nutrients. 2024. PMID: 39408292 Free PMC article. Clinical Trial. - Lactiplantibacillus plantarum P470 Isolated from Fermented Chinese Chives Has the Potential to Improve In Vitro the Intestinal Microbiota and Biological Activity in Feces of Coronary Heart Disease (CHD) Patients.
Yang L, Wu Y, Yang J, Li Y, Zhao X, Liang T, Li L, Jiang T, Zhang T, Zhang J, Zhong H, Xie X, Wu Q. Yang L, et al. Nutrients. 2024 Sep 2;16(17):2945. doi: 10.3390/nu16172945. Nutrients. 2024. PMID: 39275259 Free PMC article. - Changes in the gut microbiota of patients with sarcopenia based on 16S rRNA gene sequencing: a systematic review and meta-analysis.
Song Q, Zhu Y, Liu X, Liu H, Zhao X, Xue L, Yang S, Wang Y, Liu X. Song Q, et al. Front Nutr. 2024 Jun 28;11:1429242. doi: 10.3389/fnut.2024.1429242. eCollection 2024. Front Nutr. 2024. PMID: 39006102 Free PMC article. - Gut-Derived Short-Chain Fatty Acids and Macrophage Modulation: Exploring Therapeutic Potentials in Pulmonary Fungal Infections.
Xie Q, Li Q, Fang H, Zhang R, Tang H, Chen L. Xie Q, et al. Clin Rev Allergy Immunol. 2024 Jun;66(3):316-327. doi: 10.1007/s12016-024-08999-z. Epub 2024 Jul 5. Clin Rev Allergy Immunol. 2024. PMID: 38965168 Review.
References
- Sawzdargo M, George SR, Nguyen T, Xu S, Kolakowski LF, O'Dowd BF. A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1. Biochem Biophys Res Commun. 1997;239:543–547. - PubMed
- Im DS. Discovery of new G protein-coupled receptors for lipid mediators. J Lipid Res. 2004;45:410–418. - PubMed
- Brown AJ, Jupe S, Briscoe CP. A family of fatty acid binding receptors. DNA Cell Biol. 2005;24:54–61. - PubMed
- Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. - PubMed
- Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous