miR-19 is a key oncogenic component of mir-17-92 - PubMed (original) (raw)
miR-19 is a key oncogenic component of mir-17-92
Virginie Olive et al. Genes Dev. 2009.
Abstract
Recent studies have revealed the importance of multiple microRNAs (miRNAs) in promoting tumorigenesis, among which mir-17-92/Oncomir-1 exhibits potent oncogenic activity. Genomic amplification and elevated expression of mir-17-92 occur in several human B-cell lymphomas, and enforced mir-17-92 expression in mice cooperates with c-myc to promote the formation of B-cell lymphomas. Unlike classic protein-coding oncogenes, mir-17-92 has an unconventional gene structure, where one primary transcript yields six individual miRNAs. Here, we functionally dissected the individual components of mir-17-92 by assaying their tumorigenic potential in vivo. Using the Emu-myc model of mouse B-cell lymphoma, we identified miR-19 as the key oncogenic component of mir-17-92, both necessary and sufficient for promoting c-myc-induced lymphomagenesis by repressing apoptosis. The oncogenic activity of miR-19 is at least in part due to its repression of the tumor suppressor Pten. Consistently, miR-19 activates the Akt-mTOR (mammalian target of rapamycin) pathway, thereby functionally antagonizing Pten to promote cell survival. Our findings reveal the essential role of miR-19 in mediating the oncogenic activity of mir-17-92, and implicate the functional diversity of mir-17-92 components as the molecular basis for its pleiotropic effects during tumorigenesis.
Figures
Figure 1.
miR-19b phenocopies the oncogenic effects of mir-17-92 in the Eμ-myc model. (A) Gene structure of the mir-17-92 polycistronic cluster. (Light-colored boxes) Pre-miRNAs; (dark-colored boxes) mature miRNAs. Homologous miRNA components are indicated by the same or similar colors. (B) mir-17-92 components belong to three miRNA families: miR-17/20a/18 (blue), miR-19 (red), and miR-92a (green). Mature miRNA sequence alignments are shown for each family. Based on sequence identity, miR-17 and miR-20a are closely related homologs, sharing significant sequence identity with miR-18a, but containing a slightly different seed (74% identity among all three). miR-19a and miR-19b differ by a single nucleotide at position 11, and are likely to regulate the same mRNA targets (96% identity). miR-92a has a unique seed sequence that distinguishes it from other components. (C) The mir-19a/20/19b subcluster accelerates _c-myc_-induced lymphomagenesis. Irradiated mice reconstituted with the Eμ-myc/+ HSPCs overexpressing miR-17, miR-18a, mir-19a/20a/19b, or a MSCV control vector were monitored weekly beginning 4 wk post-transplantation. The Kaplan-Meier curves represent percentage of overall survival. (D) miR-19b accelerates _c-myc_-induced lymphomagenesis. The mir-19a/20/19b subcluster was further divided into miR-19b and miR-20a, each overexpressed in the Eμ-myc/+ HSPCs before transplantation into lethally irradiated recipient animals. Reconstituted mice were monitored weekly starting 4 wk post-transplantation. The Kaplan-Meier curve indicates miR-19b has a strong oncogenic effect.
Figure 2.
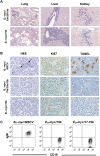
Eμ-myc/17-19 and Eμ-myc/19b lymphomas have similar pathological and immunological features. (A) Eμ-myc/19b lymphomas are highly invasive. H&E staining of the liver, lung, and kidney showed aggressive invasion by Eμ-myc/19b tumor cells, which was highly analogous to that of the Eμ-myc/19a/20a/19b lymphomas. In particular, both perivascular and parenchymal invasion of the liver were observed. (B) Overexpression of miR-19b represses _c-myc_-induced apoptosis. The Eμ-myc/19b and Eμ-myc/19a/20a/19b lymphomas had similar proliferation rates to those of Eμ-myc/MSCV controls, demonstrated by Ki67 staining. However, exogenous expression of miR-19b or mir-19a/20/19b greatly decreased apoptosis in the Eμ-myc tumors, confirmed by TUNEL and H&E staining of Eμ-myc/17-19 lymph node tumors. The “starry sky” morphology of cell clusters undergoing apoptosis (black arrows), a hallmark of Eμ-myc/MSCV lymphomas, was absent in Eμ-myc/19b and Eμ-myc/19a/20a/19b tumors. (C) miR-19b and mir-17-19b preferably transform progenitor B cells. Flow cytometric immunophenotyping of representative Eμ-myc, Eμ-myc/17-19, and Eμ-myc/19 lymphomas. While the majority of Eμ-myc tumors consisted of CD19-positive and IgM-positive B cells, the Eμ-myc/19b and Eμ-myc/17-19 tumor cells bore cellular characteristics of progenitor B cells, positive for CD19 but not for surface IgM.
Figure 3.
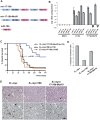
miR-19 miRNAs are essential for the oncogenic activity of mir-17-19b. (A) A schematic representation of the gene structural organization of mir-17-19b, mir-17-19b-Mut19, and miR-19b. 19a* and 19b* indicate miR-19 mutations. (B) miR-19 mutations specifically affected miR-19 expression in mir-17-19b. 3T3 cells were infected with MSCV-mir-17-19b, MSCV-mir-17-19b-Mut19, or control MSCV vectors. Expression levels of miR-17, miR-18a, miR-19a, miR-20a, and miR-19b were determined using TaqMan miRNA assays. miR-19 mutations specifically affected the expression of miR-19a and miR-19b, but not that of the adjacent mir-17-19b components. Error bars indicate SD (n = 3). (C) miR-19 is both necessary and sufficient for the oncogenic effect of mir-17-19b. Overexpression of miR-19b and mir-17-19b accelerated _c-myc_-induced lymphomagenesis to a similar degree, shown in Kaplan-Meier curves as the percentage of overall survival. Mutations in miR-19 greatly decreased the oncogenic activity of mir-17-19b in the Eμ-myc model. We compared the oncogenic effects of mir-17-19b, miR-19b, and mir-17-19b-Mut19 in the same adoptive transfer experiment. (D) miR-19 is both necessary and sufficient for the cell survival effect of mir-17-19b in vivo. Representative lymphomas from Eμ-myc, Eμ-myc/19b, and Eμ-myc/17-19b-Mut19 were stained for H&E and caspase-3, which indicated that the miR-19 mutations significantly compromised cell survival effects of mir-17-19b, while miR-19b overexrepssion suppresses apoptosis. The Eμ-myc/19b and Eμ-myc/17-19b-Mut19 tumors shown here were both IgM-negative B lymphomas. (Arrow) “Starry sky” feature of apoptotic tumor cells; (arrowhead) apoptotic cells with positive caspase-3 staining. Bar, 50 μm. (E) Quantification of caspase-3 staining of representative tumors from D as percentage of positive cells. For Eμ-myc/19b and Eμ-myc/17-19b-Mut19 tumors, only IgM-negative tumors were selected for this comparison (n = 3; error bars represent SEM).
Figure 4.
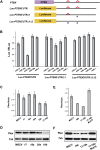
Pten is a mir-17-19b target specifically regulated by miR-19. (A) Schematic representation of the PTEN 3′UTR and its _miR-19_-binding sites. Two _miR-19_-binding sites (shown in red) can be found in the human PTEN 3′UTR. The PTEN 3′UTR with mutations (designated with asterisks) at one (_PTEN_3′UTRΔ1) or both (_PTEN_3′UTRΔ1Δ2) miR-19 sites, as well as the wild-type counterpart (_PTEN_3′UTR), were each cloned downstream from a luciferase reporter (Luc). (B) Specific repression of _Luc-PTEN_3′UTR reporter by miR-19. _Luc-PTEN_3′UTR was cotransfected with mimics of miR-17, miR-18a, miR-19b, miR-20a, and control miR-1. Only miR-19b significantly repressed the reporter expression. Cotransfection with a luciferase construct carrying one mutated miR-19b site in the PTEN 3′UTR (Luc-_PTEN_3′UTRΔ1) partially derepressed the Luc reporter, and cotransfection of a construct with mutations in both miR-19 sites (_Luc-PTEN_3′UTRΔ1Δ2) completely derepressed the Luc reporter. (C,D) miR-19b specifically represses endogenous Pten expression level. Using real-time PCR analysis (C) and Western analysis (D), down-regulation of endogenous Pten mRNA and protein can be detected in serum-starved NIH-3T3 cells infected with miR-19b and mir-17-19b. In comparison, overexpression of miR-17, miR-18a, miR-20a, and control vector (MSCV) in these cells has minimal effects on the endogenous Pten level. (E,F) miR-19 is both necessary and sufficient to mediate the Pten repression by mir-17-19b. Repression of endogenous Pten mRNA (E) and protein (F) can be detected in serum-starved NIH-3T3 cells infected with miR-19b and mir-17-19b. In comparison, mir-17-19b-Mut19 failed to repress the endogenous Pten level.
Figure 5.
miR-19b functionally antagonizes _pten_-induced apoptosis in Xenopus embryos. (A,B) miR-19b rescues hydroxyurea (HU)-induced apoptosis in X. laevis embryos. Injection of miR-19b mimics into X. laevis embryos rescued apoptosis caused by hydroxyurea treatment. The mutated miR-19b with an altered seed region (mut-19b) failed to rescue hydroxyurea-induced apoptosis. Embryos undergoing apoptosis were marked by cell blebbing, disruption of cell adhesion, and a characteristic white color (red arrowhead). Hydroxyurea-treated embryos appeared more pigmented than control embryos, largely due to developmental arrest. (B) Apoptosis was quantified for untreated and hydroxyurea-treated embryos, as well as the hydroxyurea-treated embryos coinjected with either miR-19b mimics or mut-19b mimics (n = 3 experiments with >25 embryos in each group; [*] P < 0.05). (_C_,_D_) Injection of _miR-19b_ rescued _pten_-induced apoptosis in _Xenopus_ embryos. Injection of full-length _pten_ mRNA into _Xenopus_ embryos led to widespread apoptosis. Injection of _miR-19b_, but not _mut-19b_, significantly rescued the proapoptotic effect of _pten_. (_E_,_F_) Disruption of base-pairing between _miR-19_ and _pten_ mRNA abolished their functional antagonism. Mutations in the _miR-19_-binding sites within the _pten_ mRNA (_pten-mut_) did not abrogate the proapoptotic effects of _pten_, but did eliminate the ability of _miR-19b_ to repress the apoptosis (_n_ = 3 experiments with >25 embryos in each group; [*] P < 0.05). All error bars represent SEM.
Figure 6.
miR-19 and mir-17-19b activates the Akt–mTOR pathway. (A) miR-19 is a key mir-17-19b component to activate the Akt–mTOR pathway. Using Western analysis, increased phospho-Akt level was detected in serum-starved 3T3 cells infected with miR-19b, but not miR-17, miR-18a, miR-20a, and the control vector (MSCV). In comparison, the overall Akt level was not affected by miR-19b. (B) miR-19 induces an increase in phosphorylation of S6 ribosomal protein. Enforced expression of miR-19b strongly promoted the S6 phosphorylation as compared with the rest of mir-17-19b components. (C,D) Enforced miR-19b or mir-17-19b expression in the Eμ-myc model led to an increased level of phospho-S6 in lymphomas. Cells derived from the Eμ-myc, Eμ-myc/19b, and Eμ-myc/17-19b lymphomas were analyzed by Western (C) and immunohistochemistry (D). Both Eμ-myc/19b and Eμ-myc/17-19b lymphomas exhibited a high level of phospho-S6, although mir-17-19b seemed to have a stronger effect. In comparison, Eμ-myc tumors exhibited a low level of phospho-S6 and more variation among different samples, possibly reflecting the differences in the secondary oncogenic lesions. In all Western analyses, tubulin (Tub) was used as a normalization control.
Comment in
- Tumorigenicity of the miR-17-92 cluster distilled.
van Haaften G, Agami R. van Haaften G, et al. Genes Dev. 2010 Jan 1;24(1):1-4. doi: 10.1101/gad.1887110. Genes Dev. 2010. PMID: 20047995 Free PMC article.
Similar articles
- A component of the mir-17-92 polycistronic oncomir promotes oncogene-dependent apoptosis.
Olive V, Sabio E, Bennett MJ, De Jong CS, Biton A, McGann JC, Greaney SK, Sodir NM, Zhou AY, Balakrishnan A, Foth M, Luftig MA, Goga A, Speed TP, Xuan Z, Evan GI, Wan Y, Minella AC, He L. Olive V, et al. Elife. 2013 Oct 15;2:e00822. doi: 10.7554/eLife.00822. Elife. 2013. PMID: 24137534 Free PMC article. - The PTEN-AKT-mTOR/RICTOR Pathway in Nasal Natural Killer Cell Lymphoma Is Activated by miR-494-3p via PTEN But Inhibited by miR-142-3p via RICTOR.
Chen HH, Huang WT, Yang LW, Lin CW. Chen HH, et al. Am J Pathol. 2015 May;185(5):1487-99. doi: 10.1016/j.ajpath.2015.01.025. Am J Pathol. 2015. PMID: 25907832 - miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer.
Cai J, Fang L, Huang Y, Li R, Yuan J, Yang Y, Zhu X, Chen B, Wu J, Li M. Cai J, et al. Cancer Res. 2013 Sep 1;73(17):5402-15. doi: 10.1158/0008-5472.CAN-13-0297. Epub 2013 Jul 15. Cancer Res. 2013. PMID: 23856247 - mir-17-92, a cluster of miRNAs in the midst of the cancer network.
Olive V, Jiang I, He L. Olive V, et al. Int J Biochem Cell Biol. 2010 Aug;42(8):1348-54. doi: 10.1016/j.biocel.2010.03.004. Epub 2010 Mar 19. Int J Biochem Cell Biol. 2010. PMID: 20227518 Free PMC article. Review. - mir-17-92: a polycistronic oncomir with pleiotropic functions.
Olive V, Li Q, He L. Olive V, et al. Immunol Rev. 2013 May;253(1):158-66. doi: 10.1111/imr.12054. Immunol Rev. 2013. PMID: 23550645 Free PMC article. Review.
Cited by
- Manipulating miRNA Expression: A Novel Approach for Colon Cancer Prevention and Chemotherapy.
Ramalingam S, Subramaniam D, Anant S. Ramalingam S, et al. Curr Pharmacol Rep. 2015 Jun 1;1(3):141-153. doi: 10.1007/s40495-015-0020-3. Curr Pharmacol Rep. 2015. PMID: 26029495 Free PMC article. - UVA influenced the SIRT1-miR-27a-5p-SMAD2-MMP1/COL1/BCL2 axis in human skin primary fibroblasts.
Jiang SB, Lu YS, Liu T, Li LM, Wang HX, Wu Y, Gao XH, Chen HD. Jiang SB, et al. J Cell Mol Med. 2020 Sep;24(17):10027-10041. doi: 10.1111/jcmm.15610. Epub 2020 Aug 13. J Cell Mol Med. 2020. PMID: 32790210 Free PMC article. - MicroRNA-17-5p post-transcriptionally regulates p21 expression in irradiated betel quid chewing-related oral squamous cell carcinoma cells.
Wu SY, Lin KC, Chiou JF, Jeng SC, Cheng WH, Chang CI, Lin WC, Wu LL, Lee HL, Chen RJ. Wu SY, et al. Strahlenther Onkol. 2013 Aug;189(8):675-83. doi: 10.1007/s00066-013-0347-9. Epub 2013 Jun 20. Strahlenther Onkol. 2013. PMID: 23780339 - The miR-17-92 cluster of microRNAs confers tumorigenicity by inhibiting oncogene-induced senescence.
Hong L, Lai M, Chen M, Xie C, Liao R, Kang YJ, Xiao C, Hu WY, Han J, Sun P. Hong L, et al. Cancer Res. 2010 Nov 1;70(21):8547-57. doi: 10.1158/0008-5472.CAN-10-1938. Epub 2010 Sep 17. Cancer Res. 2010. PMID: 20851997 Free PMC article. - MicroRNA in the adaptive immune system, in sickness and in health.
Liston A, Linterman M, Lu LF. Liston A, et al. J Clin Immunol. 2010 May;30(3):339-46. doi: 10.1007/s10875-010-9378-5. Epub 2010 Feb 27. J Clin Immunol. 2010. PMID: 20191314 Review.
References
- Abubaker J, Bavi PP, Al-Harbi S, Siraj AK, Al-Dayel F, Uddin S, Al-Kuraya K. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007;21:2368–2370. - PubMed
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. - PubMed
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous