A small-cell lung cancer genome with complex signatures of tobacco exposure - PubMed (original) (raw)
. 2010 Jan 14;463(7278):184-90.
doi: 10.1038/nature08629. Epub 2009 Dec 16.
Philip J Stephens, Sarah O'Meara, David J McBride, Alison Meynert, David Jones, Meng-Lay Lin, David Beare, King Wai Lau, Chris Greenman, Ignacio Varela, Serena Nik-Zainal, Helen R Davies, Gonzalo R Ordoñez, Laura J Mudie, Calli Latimer, Sarah Edkins, Lucy Stebbings, Lina Chen, Mingming Jia, Catherine Leroy, John Marshall, Andrew Menzies, Adam Butler, Jon W Teague, Jonathon Mangion, Yongming A Sun, Stephen F McLaughlin, Heather E Peckham, Eric F Tsung, Gina L Costa, Clarence C Lee, John D Minna, Adi Gazdar, Ewan Birney, Michael D Rhodes, Kevin J McKernan, Michael R Stratton, P Andrew Futreal, Peter J Campbell
Affiliations
- PMID: 20016488
- PMCID: PMC2880489
- DOI: 10.1038/nature08629
A small-cell lung cancer genome with complex signatures of tobacco exposure
Erin D Pleasance et al. Nature. 2010.
Abstract
Cancer is driven by mutation. Worldwide, tobacco smoking is the principal lifestyle exposure that causes cancer, exerting carcinogenicity through >60 chemicals that bind and mutate DNA. Using massively parallel sequencing technology, we sequenced a small-cell lung cancer cell line, NCI-H209, to explore the mutational burden associated with tobacco smoking. A total of 22,910 somatic substitutions were identified, including 134 in coding exons. Multiple mutation signatures testify to the cocktail of carcinogens in tobacco smoke and their proclivities for particular bases and surrounding sequence context. Effects of transcription-coupled repair and a second, more general, expression-linked repair pathway were evident. We identified a tandem duplication that duplicates exons 3-8 of CHD7 in frame, and another two lines carrying PVT1-CHD7 fusion genes, indicating that CHD7 may be recurrently rearranged in this disease. These findings illustrate the potential for next-generation sequencing to provide unprecedented insights into mutational processes, cellular repair pathways and gene networks associated with cancer.
Figures
Figure 1
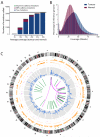
The compendium of somatic mutations in a small cell lung cancer genome. (A) Power calculations showing the number of true somatic substitutions detected (blue) and mis-calls (SNPs called as somatic mutations, burgundy, and sequencing errors called as mutations, green) for different levels of sequence coverage. Calculations are based on a true mutation prevalence of 1/Mb (black line). (B) Histogram of the actual coverage achieved per base of the tumour (blue) and normal (burgundy) genomes. (C) Figurative representation of the catalogue of somatic mutations in the genome of NCI-H209. Chromosome ideograms are shown around the outer ring and are oriented pter-qter in a clockwise direction with centromeres indicated in red. Other tracks contain somatic alterations: validated insertions (light green rectangles); validated deletions (dark green rectangles); heterozygous (light orange bars) and homozygous (dark orange bars) substitutions shown by density per 10 megabases; coding substitutions (coloured squares; silent in grey, missense in purple, nonsense in red and splice site in black); copy number (blue lines); validated intrachromosomal rearrangements (green lines); validated interchromosomal rearrangements (purple lines).
Figure 2
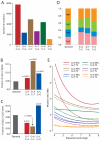
The mutation profile of NCI-H209. (A) Numbers of mutations in each of the 6 possible mutation classes. (B) Fraction of the three classes of guanine mutations occurring at CpG dinucleotides in NCI-H209, with p values reflecting the comparison with the expected fraction across the genome (grey). (C) Fraction of guanine mutations at CpGs which are found in CpG islands for each of the three classes of mutation. P values reflect comparison with the genome-wide fraction (grey) of CpGs found in CpG islands (and hence more likely to be constitutively unmethylated) versus outside CpG islands (high rates of constitutive methylation). (D) Distribution of the four NpA dinucleotides for each of the three classes of adenine mutation in NCI-H209, compared to the expected distribution across the genome (left). (E) Fitted curves showing the effects of gene expression and strand bias on mutation prevalence for the six classes of adenine and guanine mutation in NCI-H209. The y axis is expressed as mutations per Mb of at-risk nucleotides, namely mutations/1,000,000 Gs for G>T.
Figure 3
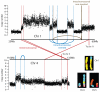
Localised complexes of somatically acquired genomic rearrangements in NCI-H209. Copy number plots across regions on chromosomes 1 and 4 are shown. Inverted intrachromosmal rearrangements (blue), non-inverted intrachromosomal rearrangements (brown) and interchromosomal rearrangements (red) are shown in relation to copy number changes. The inset shows the representative chromosomes on spectral karyotyping. There are three breakpoints between chromosome 1 (yellow) and 4 (light blue), and a translocation between chromosomes 4 and 5 (tan).
Figure 4
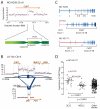
CHD7 rearrangements in SCLC cell lines. (A) A somatically acquired 39.5kb tandem duplication is found in NCI-H209. (B) The LU-135 cell line shows co-amplification of the 3′ portion of CHD7 together with MYC and the 5′ portion of PVT1. Blue lines show locations of genomic rearrangements observed in the amplicons, with the thickness of the line proportional to the number of reads spanning the breakpoint. (C) Transcripts resulting from CHD7 rearrangements are an in-frame duplication of exons 3-8 in NCI-H209 and two amplified PVT1-CHD7 fusion genes in NCI-H2171 and LU-135. (D) CHD7 is over-expressed in SCLC compared to both non-small cell lung cancer and other tumour types. LU-135 and NCI-H2171 show massive over-expression of CHD7 in keeping with the genomic amplification present in these cell lines.
References
- Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer. 2009;9:655–64. - PubMed
- Pfeifer GP, et al. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–51. - PubMed
- DeMarini DM. Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res. 2004;567:447–74. - PubMed
- Rodin SN, Rodin AS. Origins and selection of p53 mutations in lung carcinogenesis. Semin Cancer Biol. 2005;15:103–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50CA70907/CA/NCI NIH HHS/United States
- 088340/WT_/Wellcome Trust/United Kingdom
- 093867/WT_/Wellcome Trust/United Kingdom
- 077012/Z/05/Z/WT_/Wellcome Trust/United Kingdom
- P50 CA070907/CA/NCI NIH HHS/United States
- 077012/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials