A novel small molecule, LLL12, inhibits STAT3 phosphorylation and activities and exhibits potent growth-suppressive activity in human cancer cells - PubMed (original) (raw)
Brian Hutzen, Pui-Kai Li, Sarah Ball, Mingxin Zuo, Stephanie DeAngelis, Elizabeth Foust, Matthew Sobo, Lauren Friedman, Deepak Bhasin, Ling Cen, Chenglong Li, Jiayuh Lin
Affiliations
- PMID: 20072652
- PMCID: PMC2805882
- DOI: 10.1593/neo.91196
A novel small molecule, LLL12, inhibits STAT3 phosphorylation and activities and exhibits potent growth-suppressive activity in human cancer cells
Li Lin et al. Neoplasia. 2010 Jan.
Abstract
Constitutive activation of signal transducer and activator of transcription 3 (STAT3) signaling is frequently detected in cancer, promoting its emergence as a promising target for cancer treatment. Inhibiting constitutive STAT3 signaling represents a potential therapeutic approach. We used structure-based design to develop a nonpeptide, cell-permeable, small molecule, termed as LLL12, which targets STAT3. LLL12 was found to inhibit STAT3 phosphorylation (tyrosine 705) and induce apoptosis as indicated by the increases of cleaved caspase-3 and poly (ADP-ribose) polymerase in various breast, pancreatic, and glioblastoma cancer cell lines expressing elevated levels of STAT3 phosphorylation. LLL12 could also inhibit STAT3 phosphorylation induced by interleukin-6 in MDA-MB-453 breast cancer cells. The inhibition of STAT3 by LLL12 was confirmed by the inhibition of STAT3 DNA binding activity and STAT3-dependent transcriptional luciferase activity. Downstream targets of STAT3, cyclin D1, Bcl-2, and survivin were also downregulated by LLL12 at both protein and messenger RNA levels. LLL12 is a potent inhibitor of cell viability, with half-maximal inhibitory concentrations values ranging between 0.16 and 3.09 microM, which are lower than the reported JAK2 inhibitor WP1066 and STAT3 inhibitor S3I-201 in six cancer cell lines expressing elevated levels of STAT3 phosphorylation. In addition, LLL12 inhibits colony formation and cell migration and works synergistically with doxorubicin and gemcitabine. Furthermore, LLL12 demonstrated a potent inhibitory activity on breast and glioblastoma tumor growth in a mouse xenograft model. Our results indicate that LLL12 may be a potential therapeutic agent for human cancer cells expressing constitutive STAT3 signaling.
Figures
Figure 1
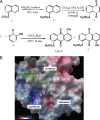
(A) Synthesis of LLL12 (includes chemical structure). (B) Computer model of LLL12 binding to the STAT3 SH2 domain. The ball-and-stick model of pTyr705-Leu706 is the binding mode of the partnering SH2 during the STAT3 homodimerization. LLL12 effectively displaces its binding through stronger binding to pTyr705 binding site, indicating LLL12 can efficiently prevent STAT3 SH2 dimerization.
Figure 2
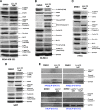
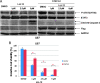
Western blot analysis of cells treated with LLL12. Cancer cell lines expressing constitutively active STAT3, (A) MDA-MB-231, (B) SK-BR-3, (C) HPAC, (D) U87, exhibit a decrease in the levels of expression of STAT3 phosphorylation after treatment with LLL12. Downstream targets of STAT3, cyclin D1, Bcl-2, and survivin, were inhibited. Apoptosis is also indicated by the induction of cleaved PARP and caspase-3. Normal cell lines that do not express elevated levels of STAT3 phosphorylation, (E) HPDE cells, HMEC, HHs, and normal human lung fibroblasts (WI-38), did not exhibit an induction of cleaved PARP or caspase-3 after treatment with LLL12.
Figure 3
LLL12 inhibits STAT3 phosphorylation induced by IL-6 in MDA-MB-453 breast cancer cells. The cells were serum-starved overnight, then left untreated or were treated with LLL12 (0.5–2 µM) orDMSO. After 2 hours, the untreated and LLL12-treated cells were stimulated by IL-6 (25 ng/ml). The cells were harvested at 30 minutes and analyzed by Western blot.
Figure 4
LLL12 has an inhibitory effect on STAT3 DNA binding activity and STAT3-dependent transcriptional activity. The nuclear extracts of (A) SK-BR-3, (B) MDA-MB-231, (C) HPAC, and (D) U87 cancer cells were analyzed for STAT3 DNA binding. STAT1 DNA binding was also looked at to demonstrate the specificity of LLL12 to STAT3 over STAT1 protein in (B) MDA-MB-231 and (D) U87 cancer cells. Statistical significance (P < .05) relative to the DMSO vehicle control is designated by an asterisk.
Figure 5
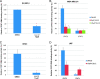
(A) STAT3-dependent transcriptional activity was analyzed in a luciferase assay. MDA-MB-231 breast cancer cloned cells that stably integrate the STAT3-dependent luciferase reporter construct, pLucTKS3, were used. Results are reported relative to a pLucTKS3-transfected sample treated with DMSO set at 100%. Statistical significance (P < .05) relative to DMSO is designated by an asterisk. (B) Transcription of STAT3-regulated genes is inhibited by LLL12. RT-PCR reveals decreased expression of STAT3 target genes over a DMSO control after treatment with LLL12.
Figure 6
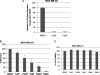
(A) Colony formation of MDA-MB-231 cells in soft agar is inhibited by LLL12. The potency of LLL12 was assessed further in an anchorage-independent environment through a colony formation assay. Treatment with LLL12 greatly decreased the ability of MDA-MB-231 cells to form colonies in comparison to a DMSO control. (B) LLL12 inhibits cell migration in MDA-MB-231 breast cancer cells. A wound healing assay reveals that LLL12 has a significant impact on MDA-MB-231 cell migration. The ability of the cells to migrate is increasingly inhibited by an increase in dose of LLL12. Statistical significance (P < .05) relative to the DMSO control is designated by an asterisk. (C) A cell viability assay (MTT) was done to determine if the effect of LLL12 on MDA-MB-231 cell migration was due to its ability to inhibit cell proliferation. The time points of treatment (4 hours with LLL12) and incubation (additional 20 hours without LLL12) used in the wound healing assay was applied in the viability assay. The ability of LLL12 to inhibit cell migration does not seem to be due to an inhibition of cell proliferation.
Figure 7
The combinatorial effect of LLL12 and chemotherapy drugs, doxorubicin and gemcitabine. (A) MDA-MB-231 breast cancer cells were treated with LLL12 and doxorubicin individually and in combination. (B) HPAC pancreatic cancer cells were treated with LLL12 and gemcitabine individually and in combination. Cell viability was determined by MTT assay. A synergistic effect between LLL12 and doxorubicin or gemcitabine is indicated by an asterisk.
Figure 8
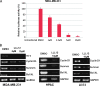
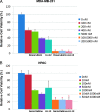
The effect of STAT3-C expression on LLL12-mediated inhibition in U87 glioblastoma cells. Cells were transfected with a vector expressing constitutively active STAT3, STAT3-C for 24 hours, then treated with LLL12 for another 24 hours. (A) LLL12-induced caspase-3 cleavage was rescued in U87 cells when STAT3-C protein was expressed. (B) The inhibition of cell viability of LLL12 in U87 cells was also reduced in the presence of STAT3-C protein in MTT assay (*P < .05).
Figure 9
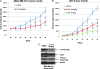
Effect of LLL12 on tumor growth in mouse xenografts with MDA-MB-231 breast cancer cells (A) or U87 glioblastoma cells (B). After tumor development, the mice were given daily intraperitoneal dosages of 2.5 to 5 mg/kg LLL12 or DMSO (*P < .05). STAT3 but not ERK1/2 phosphorylation of MDA-MB-231 tumor tissue samples from these mice was also decreased (C).
Similar articles
- The small molecule, LLL12, inhibits STAT3 phosphorylation and induces apoptosis in medulloblastoma and glioblastoma cells.
Ball S, Li C, Li PK, Lin J. Ball S, et al. PLoS One. 2011 Apr 19;6(4):e18820. doi: 10.1371/journal.pone.0018820. PLoS One. 2011. PMID: 21526200 Free PMC article. - LLL12, a novel small inhibitor targeting STAT3 for hepatocellular carcinoma therapy.
Zuo M, Li C, Lin J, Javle M. Zuo M, et al. Oncotarget. 2015 May 10;6(13):10940-9. doi: 10.18632/oncotarget.3458. Oncotarget. 2015. PMID: 25883212 Free PMC article. - XZH-5 inhibits STAT3 phosphorylation and enhances the cytotoxicity of chemotherapeutic drugs in human breast and pancreatic cancer cells.
Liu A, Liu Y, Jin Z, Hu Q, Lin L, Jou D, Yang J, Xu Z, Wang H, Li C, Lin J. Liu A, et al. PLoS One. 2012;7(10):e46624. doi: 10.1371/journal.pone.0046624. Epub 2012 Oct 3. PLoS One. 2012. PMID: 23056374 Free PMC article. - Targeting STAT3 Enzyme for Cancer Treatment.
Arun S, Patel PK, Lakshmanan K, Rajangopal K, Swaminathan G, Byran G. Arun S, et al. Mini Rev Med Chem. 2024;24(13):1252-1261. doi: 10.2174/0113895575254012231024062619. Mini Rev Med Chem. 2024. PMID: 38299278 Review. - Increasing the range of drug targets: interacting peptides provide leads for the development of oncoprotein inhibitors.
Groner B, Weber A, Mack L. Groner B, et al. Bioengineered. 2012 Nov-Dec;3(6):320-5. doi: 10.4161/bioe.21272. Epub 2012 Jul 24. Bioengineered. 2012. PMID: 22825353 Free PMC article. Review.
Cited by
- Hirsutinolide Series Inhibit Stat3 Activity, Alter GCN1, MAP1B, Hsp105, G6PD, Vimentin, TrxR1, and Importin α-2 Expression, and Induce Antitumor Effects against Human Glioma.
Miklossy G, Youn UJ, Yue P, Zhang M, Chen CH, Hilliard TS, Paladino D, Li Y, Choi J, Sarkaria JN, Kawakami JK, Wongwiwatthananukit S, Chen Y, Sun D, Chang LC, Turkson J. Miklossy G, et al. J Med Chem. 2015 Oct 8;58(19):7734-48. doi: 10.1021/acs.jmedchem.5b00686. Epub 2015 Sep 17. J Med Chem. 2015. PMID: 26331426 Free PMC article. - Salinomycin Promotes Anoikis and Decreases the CD44+/CD24- Stem-Like Population via Inhibition of STAT3 Activation in MDA-MB-231 Cells.
An H, Kim JY, Oh E, Lee N, Cho Y, Seo JH. An H, et al. PLoS One. 2015 Nov 3;10(11):e0141919. doi: 10.1371/journal.pone.0141919. eCollection 2015. PLoS One. 2015. PMID: 26528725 Free PMC article. - MicroRNA-218 functions as a tumor suppressor in lung cancer by targeting IL-6/STAT3 and negatively correlates with poor prognosis.
Yang Y, Ding L, Hu Q, Xia J, Sun J, Wang X, Xiong H, Gurbani D, Li L, Liu Y, Liu A. Yang Y, et al. Mol Cancer. 2017 Aug 22;16(1):141. doi: 10.1186/s12943-017-0710-z. Mol Cancer. 2017. PMID: 28830450 Free PMC article. - Hydroxamic Acid and Benzoic Acid-Based STAT3 Inhibitors Suppress Human Glioma and Breast Cancer Phenotypes In Vitro and In Vivo.
Yue P, Lopez-Tapia F, Paladino D, Li Y, Chen CH, Namanja AT, Hilliard T, Chen Y, Tius MA, Turkson J. Yue P, et al. Cancer Res. 2016 Feb 1;76(3):652-63. doi: 10.1158/0008-5472.CAN-14-3558. Epub 2015 Jun 18. Cancer Res. 2016. PMID: 26088127 Free PMC article.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
- Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. p 1. - PubMed
- Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494–503. quiz 1 p following 516. - PubMed
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous