Modelling event-related skin conductance responses - PubMed (original) (raw)
Modelling event-related skin conductance responses
Dominik R Bach et al. Int J Psychophysiol. 2010 Mar.
Abstract
Analytic tools for psychophysiological signals often make implicit assumptions that are unspecified. In developing a mathematical framework for analysis of skin conductance responses [SCRs], we formalise our assumptions by positing that SCRs can be regarded as the output of a linear time-invariant filter. Here, we provide an empirical test of these assumptions. Our findings indicate that a large component of the variance in SCRs can be explained by one response function per individual. We note that baseline variance (i.e. variance in the absence of evoked responses) is higher than variance that could not be explained by a linear time-invariant model of evoked responses. Furthermore, there was no evidence for nonlinear interactions among evoked responses that depended on their temporal overlap. We develop a canonical response function and show that it can be used for signals from different recording sites. We discuss the implications of these observations for model-based analysis of SCRs.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures
Fig. 1
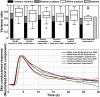
Variance partitioning and response functions were estimated for different stimulus classes. Top panel: bar charts show variance components across subjects; that is, variance explained by one common response function (black), between-subjects variance (grey) and residual variance (white) for each experiment. Light grey: baseline variance in the absence of evoked responses for the visual detection experiment. Box plots depict explained variance within subjects, showing median (line), quartiles (box), and range (whiskers). Outliers (values outside the 1.5 × interquartile range) are shown as individual dots; whiskers then represent the data within the interquartile range. Bottom panel: empirical (PCA) response functions across participants for the different experiments.
Fig. 2
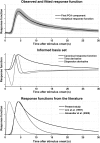
A canonical response function was derived from observed responses (shown as the first PCA component ± standard deviation across all observations), and was described analytically as an exponentially modified Gaussian function (top panel). Time and dispersion derivatives were constructed to account for differences in response shape between participants and experimental conditions (middle panel); the complete basis set could explain 64.0% of the total variance. Latency and rise time are comparable to the function proposed by Lim et al. (1997).
Fig. 3
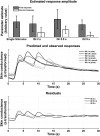
The linearity assumption was tested by presenting either single white noise sounds, or a sequence of two sounds with differing ISIs, separated by silent periods of 30–40 s. For each individual, a response function was estimated from their responses to single events, and responses to double events were estimated under time-invariance assumptions. It turns out that responses to second events are smaller than to the first event in each sequence, regardless of ISI (2–9 s); however, there is no evidence for dependency of the repetition suppression on ISI. Averaged residuals are similar for the first and second event, thus indicating no systematic alterations of response shape at different ISIs.
Fig. 4
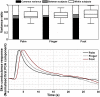
Variance portioning and response functions for hand (thenar/hypothenar), finger (volar surface, 2nd/3rd finger, middle phalanx), and medial foot (medial plantar) recordings. The ratio of explained variance is similar, and the response functions mainly differ in peak latency. Note that the canonical response function depicted in Fig. 2 can explain responses from all three recording sites almost equally well.
Similar articles
- Effects of inter-stimulus interval on skin conductance responses and event-related potentials in a Go/NoGo task.
Recio G, Schacht A, Sommer W. Recio G, et al. Biol Psychol. 2009 Feb;80(2):246-50. doi: 10.1016/j.biopsycho.2008.10.007. Epub 2008 Oct 31. Biol Psychol. 2009. PMID: 19022336 - Effortless control: executive attention and conscious feeling of mental effort are dissociable.
Naccache L, Dehaene S, Cohen L, Habert MO, Guichart-Gomez E, Galanaud D, Willer JC. Naccache L, et al. Neuropsychologia. 2005;43(9):1318-28. doi: 10.1016/j.neuropsychologia.2004.11.024. Epub 2005 Jan 20. Neuropsychologia. 2005. PMID: 15949516 - EEG and event related potentials in hepatic encephalopathy.
Davies MG, Rowan MJ, Feely J. Davies MG, et al. Metab Brain Dis. 1991 Dec;6(4):175-86. doi: 10.1007/BF00996917. Metab Brain Dis. 1991. PMID: 1812391 Review. - The clinical role of evoked potentials.
Walsh P, Kane N, Butler S. Walsh P, et al. J Neurol Neurosurg Psychiatry. 2005 Jun;76 Suppl 2(Suppl 2):ii16-22. doi: 10.1136/jnnp.2005.068130. J Neurol Neurosurg Psychiatry. 2005. PMID: 15961863 Free PMC article. Review. No abstract available.
Cited by
- Neuroticism/Negative Emotionality Is Associated with Increased Reactivity to Uncertain Threat in the Bed Nucleus of the Stria Terminalis, Not the Amygdala.
Grogans SE, Hur J, Barstead MG, Anderson AS, Islam S, Kim HC, Kuhn M, Tillman RM, Fox AS, Smith JF, DeYoung KA, Shackman AJ. Grogans SE, et al. J Neurosci. 2024 Aug 7;44(32):e1868232024. doi: 10.1523/JNEUROSCI.1868-23.2024. J Neurosci. 2024. PMID: 39009438 - Autonomic and brain responses associated with empathy deficits in autism spectrum disorder.
Gu X, Eilam-Stock T, Zhou T, Anagnostou E, Kolevzon A, Soorya L, Hof PR, Friston KJ, Fan J. Gu X, et al. Hum Brain Mapp. 2015 Sep;36(9):3323-38. doi: 10.1002/hbm.22840. Epub 2015 May 21. Hum Brain Mapp. 2015. PMID: 25995134 Free PMC article. - Mapping Grip Force Characteristics in the Measurement of Stress in Driving.
Sahar Y, Elbaum T, Musicant O, Wagner M, Altarac L, Shoval S. Sahar Y, et al. Int J Environ Res Public Health. 2023 Feb 23;20(5):4005. doi: 10.3390/ijerph20054005. Int J Environ Res Public Health. 2023. PMID: 36901016 Free PMC article. - Abnormal autonomic and associated brain activities during rest in autism spectrum disorder.
Eilam-Stock T, Xu P, Cao M, Gu X, Van Dam NT, Anagnostou E, Kolevzon A, Soorya L, Park Y, Siller M, He Y, Hof PR, Fan J. Eilam-Stock T, et al. Brain. 2014 Jan;137(Pt 1):153-71. doi: 10.1093/brain/awt294. Brain. 2014. PMID: 24424916 Free PMC article. - Generalization of learned pain modulation depends on explicit learning.
Koban L, Kusko D, Wager TD. Koban L, et al. Acta Psychol (Amst). 2018 Mar;184:75-84. doi: 10.1016/j.actpsy.2017.09.009. Epub 2017 Oct 10. Acta Psychol (Amst). 2018. PMID: 29025685 Free PMC article.
References
- Alexander D.M., Trengove C., Johnston P., Cooper T., August J.P., Gordon E. Separating individual skin conductance responses in a short interstimulus-interval paradigm. J. Neurosci. Methods. 2005;146:116–123. - PubMed
- Barry R.J., Feldmann S., Gordon E., Cocker K.I., Rennie C. Elicitation and habituation of the electrodermal orienting response in a short interstimulus interval paradigm. Int. J. Psychophysiol. 1993;15:247–253. - PubMed
- Boucsein W. Electrodermal Activity. Springer; Berlin: 1992.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources