IPS-1 is essential for the control of West Nile virus infection and immunity - PubMed (original) (raw)
. 2010 Feb 5;6(2):e1000757.
doi: 10.1371/journal.ppat.1000757.
Daphne Y Ma, Sunil Thomas, Jennifer M Lund, Nu Zhang, Stephane Daffis, Alexander Y Rudensky, Michael J Bevan, Edward A Clark, Murali-Krishna Kaja, Michael S Diamond, Michael Gale Jr
Affiliations
- PMID: 20140199
- PMCID: PMC2816698
- DOI: 10.1371/journal.ppat.1000757
IPS-1 is essential for the control of West Nile virus infection and immunity
Mehul S Suthar et al. PLoS Pathog. 2010.
Abstract
The innate immune response is essential for controlling West Nile virus (WNV) infection but how this response is propagated and regulates adaptive immunity in vivo are not defined. Herein, we show that IPS-1, the central adaptor protein to RIG-I-like receptor (RLR) signaling, is essential for triggering of innate immunity and for effective development and regulation of adaptive immunity against pathogenic WNV. IPS-1(-/-) mice exhibited increased susceptibility to WNV infection marked by enhanced viral replication and dissemination with early viral entry into the CNS. Infection of cultured bone-marrow (BM) derived dendritic cells (DCs), macrophages (Macs), and primary cortical neurons showed that the IPS-1-dependent RLR signaling was essential for triggering IFN defenses and controlling virus replication in these key target cells of infection. Intriguingly, infected IPS-1(-/-) mice displayed uncontrolled inflammation that included elevated systemic type I IFN, proinflammatory cytokine and chemokine responses, increased numbers of inflammatory DCs, enhanced humoral responses marked by complete loss of virus neutralization activity, and increased numbers of virus-specific CD8+ T cells and non-specific immune cell proliferation in the periphery and in the CNS. This uncontrolled inflammatory response was associated with a lack of regulatory T cell expansion that normally occurs during acute WNV infection. Thus, the enhanced inflammatory response in the absence of IPS-1 was coupled with a failure to protect against WNV infection. Our data define an innate/adaptive immune interface mediated through IPS-1-dependent RLR signaling that regulates the quantity, quality, and balance of the immune response to WNV infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
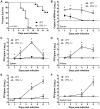
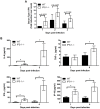
Figure 1. Virologic analysis in wild type and IPS-1−/− mice.
Adult wild type and IPS-1−/− mice were infected s.c. with 100 PFU of WN-TX. (A) Differential lethality from WNV infection (WT n = 13; IPS-1−/− n = 23; p<0.0001). B–F. Viral burden analysis of peripheral and CNS tissues from wild type and IPS-1−/− mice infected s.c. with 100 PFU of WN-TX. (B) WNV RNA in serum and infectious virus in the (C) spleen, (D) kidney, (E) brain, and (F) spinal cord were determined by RT-qPCR assay (B) or viral plaque assay (C–F) of samples harvested on day 1, 2, 4, and 6 pi. Data are shown as copies of WNV genome RNA per ml of serum or PFU per gram of tissue for 3 to 6 mice per timepoint. Graphs show the mean +/− standard deviation for each measurement. Asterisk denotes p<0.05. The horizontal line indicates the lower limit of assay sensitivity.
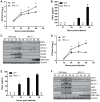
Figure 2. IPS-1 is essential for triggering the innate immune response to WNV infection and controlling virus replication in myeloid cells.
Primary bone-marrow derived dendritic cells (A–C) and macrophages (D–F) recovered from wild type mice and IPS-1−/− mice were mock-infected (M) or infected with WN-TX at an MOI of 1.0. Cells and culture media were harvested at the times indicated pi for determination of virus load (A, D) and IFN-β production (B, E), respectively. n.d. = not detected. Graphs show the mean +/− standard deviation from three independent experiments. Asterisk denotes p<0.05. (C,F) Immunoblot analysis of protein abundance in lysates from mock-infected (M) and WN-TX infected cells. For panel C, STAT-1 expression in dendritic cells was normalized at each timepoint to loading control and compared to mock (relative fold induction WT, KO at 12 hours 1.72, 0.7; 24 hours 2.6, 0.8; 36 hours 4.6, 1.1; 48 hours 9.9, 0.6).
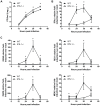
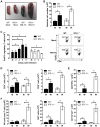
Figure 3. IPS-1 is essential for triggering innate immune defenses and controlling virus replication during WNV infection of primary cortical neurons.
Primary cortical neurons (CN) from WT and IPS-1−/− mice were infected at an MOI of 1.0. (A) Viral titers in the culture supernatants were determined by plaque assay. (B, C) mRNA expression was determined by RT-qPCR assay using primers-specific for (B) IFN-β or (C) ISG56, ISG49, RIG-I, and MDA5. Graphs show the mean +/− standard deviation from triplicate independent analyses. Asterisks denote p<0.05.
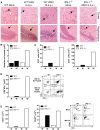
Figure 4. Increased CNS inflammation in WNV-infected IPS-1−/− mice.
(A) H&E stained saggital brain tissue sections. The arrows denote areas of interest. (B–E) Brain leukocytes were recovered from wild type and IPS-1−/− mice six days pi. (B) The total number of brain lymphocytes was determined by cell counting. (C) Total CD4+ (left) and CD8+ T cells (right), (D) Total WNV-specific CD8+ T cells (left) with a representative analysis of the frequency of TNF-α and IFN-γ expression within brain CD8+ cells (right left), and (E) Total number of microglia/infiltrating macrophages (left) were determined by flow cytometry (right; representative analysis). M = Mock; W = WN-TX. Data are representative of two or more independent experiments, and each analysis represents a pool of 5 mouse brains.
Figure 5. Enhanced levels of IFN, proinflammatory cytokines, and chemokines in serum from WNV-infected IPS-1−/− mice.
Serum was collected from wild type and IPS-1−/− mice. (A) Type I IFN levels at 1, 2, and 4 days pi. (B) Proinflammatory cytokines and chemokines were measured on days 1 and 4 pi. Graphs show the mean +/− standard deviation from triplicate measurements of duplicate experiments. Asterisks denote p<0.001.
Figure 6. Immune cell subsets within the popliteal draining lymph node during acute WNV infection.
Wild type and IPS-1−/− mice were mock-infected (M) or infected with WN-TX (W), and popliteal draining lymph nodes were harvested 24 hr later. Lymphoid cells were isolated and cells were analyzed by flow cytometry. (A–C) total numbers of cells expressing various dendritic cell surface markers, (D) plasmacytoid cell surface markers (CD11cint/lo/B220+/siglec H+). (E and F) T cells (G) B cells. A representative flow cytometric analysis of the CD11c+/CD11bhi/Ly6C+ DC subset is shown in C (left panel set). Data show the mean +/− standard deviation from triplicate samples of duplicate experiments. Asterisks denote p<0.05.
Figure 7. Enhanced inflammation in IPS-1−/− infected mice associates with a lack of Treg expansion during WNV infection.
Wild type and IPS-1−/− mice were mock-infected (M) or infected with WN-TX (W). Spleens were harvested and immune cells were isolated, counted, and characterized by flow cytometry. (A) Spleen morphology. (B) Absolute cell counts. (C) Total CD4+/FoxP3+ regulatory T cells (left; M = mock) and representative flow cytometric analysis of cell frequency from samples on day 6 pi (right). (D) CD4+ and CD8+ T cells. (E) NS4B antigen-specific CD8+ T cells. (F) CD4−/CD8−/NK1.1+ NK cells, CD4+/CD8+/NK1.1+ NKT cells, and CD11c+/Gr1+ neutrophils. Graphs show the mean +/− standard deviation from triplicate samples of duplicate experiments. Asterisks denote p<0.05.
Similar articles
- Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7.
Daffis S, Suthar MS, Szretter KJ, Gale M Jr, Diamond MS. Daffis S, et al. PLoS Pathog. 2009 Oct;5(10):e1000607. doi: 10.1371/journal.ppat.1000607. Epub 2009 Oct 2. PLoS Pathog. 2009. PMID: 19798431 Free PMC article. - Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1.
Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M Jr. Fredericksen BL, et al. J Virol. 2008 Jan;82(2):609-16. doi: 10.1128/JVI.01305-07. Epub 2007 Oct 31. J Virol. 2008. PMID: 17977974 Free PMC article. - The innate immune adaptor molecule MyD88 restricts West Nile virus replication and spread in neurons of the central nervous system.
Szretter KJ, Daffis S, Patel J, Suthar MS, Klein RS, Gale M Jr, Diamond MS. Szretter KJ, et al. J Virol. 2010 Dec;84(23):12125-38. doi: 10.1128/JVI.01026-10. Epub 2010 Sep 29. J Virol. 2010. PMID: 20881045 Free PMC article. - Immunology of West Nile Virus Infection and the Role of Alpha-Synuclein as a Viral Restriction Factor.
Lesteberg KE, Beckham JD. Lesteberg KE, et al. Viral Immunol. 2019 Jan/Feb;32(1):38-47. doi: 10.1089/vim.2018.0075. Epub 2018 Sep 15. Viral Immunol. 2019. PMID: 30222521 Review. - The innate immune playbook for restricting West Nile virus infection.
Quicke KM, Suthar MS. Quicke KM, et al. Viruses. 2013 Oct 30;5(11):2643-58. doi: 10.3390/v5112643. Viruses. 2013. PMID: 24178712 Free PMC article. Review.
Cited by
- Pathogenic Chikungunya Virus Evades B Cell Responses to Establish Persistence.
Hawman DW, Fox JM, Ashbrook AW, May NA, Schroeder KMS, Torres RM, Crowe JE Jr, Dermody TS, Diamond MS, Morrison TE. Hawman DW, et al. Cell Rep. 2016 Aug 2;16(5):1326-1338. doi: 10.1016/j.celrep.2016.06.076. Epub 2016 Jul 21. Cell Rep. 2016. PMID: 27452455 Free PMC article. - Increased early RNA replication by chimeric West Nile virus W956IC leads to IPS-1-mediated activation of NF-κB and insufficient virus-mediated counteraction of the resulting canonical type I interferon signaling.
Scherbik SV, Pulit-Penaloza JA, Basu M, Courtney SC, Brinton MA. Scherbik SV, et al. J Virol. 2013 Jul;87(14):7952-65. doi: 10.1128/JVI.02842-12. Epub 2013 May 15. J Virol. 2013. PMID: 23678179 Free PMC article. - Characterization of flavivirus infection in salivary gland cultures from male Ixodes scapularis ticks.
Kendall BL, Grabowski JM, Rosenke R, Pulliam M, Long DR, Scott DP, Offerdahl DK, Bloom ME. Kendall BL, et al. PLoS Negl Trop Dis. 2020 Oct 5;14(10):e0008683. doi: 10.1371/journal.pntd.0008683. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33017410 Free PMC article. - West Nile Virus: biology, transmission, and human infection.
Colpitts TM, Conway MJ, Montgomery RR, Fikrig E. Colpitts TM, et al. Clin Microbiol Rev. 2012 Oct;25(4):635-48. doi: 10.1128/CMR.00045-12. Clin Microbiol Rev. 2012. PMID: 23034323 Free PMC article. Review. - RIG-I like receptors in antiviral immunity and therapeutic applications.
Ireton RC, Gale M Jr. Ireton RC, et al. Viruses. 2011 Jun;3(6):906-19. doi: 10.3390/v3060906. Epub 2011 Jun 23. Viruses. 2011. PMID: 21994761 Free PMC article. Review.
References
- Cdc. West Nile virus activity–United States, 2007. MMWR. 2008;57:720–723. - PubMed
- Debiasi RL, Parsons JA, Grabert BE. West Nile virus meningoencephalitis in an immunocompetent adolescent. Pediatr Neurol. 2005;33:217–219. - PubMed
- Emig M, Apple DJ. Severe West Nile virus disease in healthy adults. Clin Infec Dis. 2004;38:289–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI083019/AI/NIAID NIH HHS/United States
- AI057568/AI/NIAID NIH HHS/United States
- T32AI007411/AI/NIAID NIH HHS/United States
- R21 AI057568/AI/NIAID NIH HHS/United States
- AI083019/AI/NIAID NIH HHS/United States
- T32 AI007411/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous