Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis - PubMed (original) (raw)
Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis
Venu M Nemani et al. Neuron. 2010.
Abstract
The protein alpha-synuclein accumulates in the brain of patients with sporadic Parkinson's disease (PD), and increased gene dosage causes a severe, dominantly inherited form of PD, but we know little about the effects of synuclein that precede degeneration. alpha-Synuclein localizes to the nerve terminal, but the knockout has little if any effect on synaptic transmission. In contrast, we now find that the modest overexpression of alpha-synuclein, in the range predicted for gene multiplication and in the absence of overt toxicity, markedly inhibits neurotransmitter release. The mechanism, elucidated by direct imaging of the synaptic vesicle cycle, involves a specific reduction in size of the synaptic vesicle recycling pool. Ultrastructural analysis demonstrates reduced synaptic vesicle density at the active zone, and imaging further reveals a defect in the reclustering of synaptic vesicles after endocytosis. Increased levels of alpha-synuclein thus produce a specific, physiological defect in synaptic vesicle recycling that precedes detectable neuropathology.
Figures
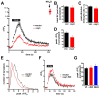
Figure 1. Over-expression of α-synuclein inhibits synaptic vesicle exocytosis
(A) Time course of changes in the fluorescence of VGLUT1-pHluorin during and after 10 Hz stimulation for 60 s in neurons cotransfected with either wild type human αsyn or empty vector. Alkalinization with 50 mM NH4Cl reveals total VGLUT1-pHluorin (arrow). n=3 coverslips, 60 boutons for each condition. (B) Total fluorescence of VGLUT1-pHluorin revealed by the addition of NH4Cl shows that expression of the reporter on all synaptic vesicles is not altered by the over-expression of αsyn. Values are normalized to the fluorescence obtained in vector control. (C) The rate of endocytosis (τ) at the end of 10 Hz stimulation was determined by the fit to a single exponential, and shows no effect of αsyn over-expression. (D) Peak ΔF/F0 normalized to vector control shows a reduction in cells over-expressing wild type αsyn. *, p<0.05 for αsyn versus control, two-tailed, unpaired t-test. For panels B–D, n=9 coverslips, 180 nerve terminals from each condition and 3 independent transfections. (E) Frequency histogram of peak ΔF/F0 from a large number of synapses in response to 10 Hz stimulation for 60 s. n=9 coverslips from 3 independent transfections, 1809 boutons for vector, and 1428 boutons for αsyn (F) Time course of VGLUT1-pHluorin fluorescence change during and after 10 Hz stimulation for 60 s in hippocampal neurons from either wild type or α-synuclein knockout mice. n=3 coverslips, 60 boutons for each condition. (G) Peak ΔF/F0 after a 10 Hz 60 s stimulus normalized to total synaptic vesicle pool size (revealed by addition of NH4Cl) shows no significant difference between wild type and either αsyn KO or α/β double KO neurons. n=6 coverslips, 120 nerve terminals from each condition with 2 independent transfections. Values represent mean ± SEM.
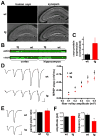
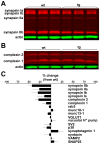
Figure 2. α–Synuclein over-expression in transgenic mice inhibits synaptic transmission
(A) Brain sections from 3 week old transgenic mice (tg) and wildtype (wt) littermates were double stained for human αsyn using the 15G7 antibody, and for synapsins using an antibody that recognizes both synapsins I and II. (B) Extracts (10 μg) from cortex or hippocampus of αsyn transgenic mice and wt littermates were immunoblotted in triplicate using an antibody to actin and the syn-1 antibody to both mouse and human αsyn. (C) Quantitation of the western analysis shown in B indicates ~3-fold over-expression of α-synuclein in the transgenic mice. Samples from each animal were loaded in triplicate, and syn-1 immunoreactivity normalized to actin detected in the same blot. n=3 animals (D) Representative fEPSP traces from CA1 stratum radiatum of 3–5 week old transgenic mice and wild type littermates in response to increasing stimulation of Schaffer collaterals (left). An input-output curve of fiber volley amplitude versus fEPSP slope shows significantly less postsynaptic response by αsyn transgenic mice than wild type littermates (right). p<0.005 for all points by two-tailed t-test; n=44 slices for wt, 48 slices for transgenic mice from 11 mice for each (E) fEPSP response in CA1 stratum radiatum to paired pulse stimulation of Schaffer collaterals with a 40 ms interstimulus interval. The left panel shows representative fEPSPs recorded from slices of wild type (top) and transgenic animals (bottom). p<0.05 by unpaired, two-tailed t-test; n=45 slices for wt and 47 for transgenic mice. (F) Transgenic over-expression of αsyn reduces the frequency of spontaneous release (right) but not mEPSC amplitude (left). Values represent mean ± SEM.
Figure 3. α–Synuclein inhibits synaptic vesicle exocytosis in midbrain dopamine neurons
(A) Postnatal midbrain neurons cotransfected with VGLUT1-pHluorin and either human αsyn or empty vector were grown for 14–21 DIV and immunostained for human αsyn using the human-specific αsyn antibody 15G7, for VGLUT1-pHluorin using a monoclonal antibody to GFP, and for tyrosine hydroxylase (TH) using a polyclonal antibody. Arrowheads indicate TH+ boutons expressing VGLUT1-pHluorin with or without human αsyn. Scale bar = 5 μm. (B) Time course of response to 10 Hz stimulation for 60 s by TH+ neurons expressing VGLUT1-pHluorin and either wild type human αsyn or empty vector. Inset, peak ΔF/F0 values normalized to the response by cells transfected with empty vector. p<0.001, n=7 coverslips, 116 nerve terminals for vector, and 8 coverslips, 84 nerve terminals for αsyn from 2 independent transfections. Values indicate mean ± SEM.
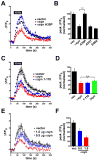
Figure 4. α–Synuclein reduces the recycling pool of synaptic vesicles
(A) Hippocampal neurons cotransfected with VGLUT1-pHluorin and either human αsyn or empty vector were stimulated at 30 Hz for 3 s to activate specifically the readily releasable pool of synaptic vesicles. n=3 coverslips, 60 boutons per condition. Inset, peak ΔF/F0 values normalized to the response in the vector control shows a substantial reduction in the cells over-expressing human αsyn. n=6 coverslips, 120 boutons per condition from 2 independent transfections. p<0.0001 by two-tailed, unpaired t-test. (B) Neurons expressing VGLUT1-pHluorin were stimulated with Tyrode’s solution containing 500 mM sucrose in the presence of 1μM bafilomycin to prevent reacidification of the internalized vesicles, and imaged in the absence of sucrose (to avoid distortion by changes in refractive index) both before and after stimulation. The change in ΔF/F0 normalized to vector control shows a reduction in neurons over-expressing human αsyn. n=9 coverslips, 180 boutons per condition from 3 independent transfections. p<0.05 by two-tailed, unpaired t-test. (C) Hippocampal neurons transfected with either αsyn-IRES2-GFP or IRES2-GFP were loaded with 15 μM FM 4-64 by 10 Hz stimulation for 60 s, maintained in the dye for 60 additional seconds to allow full endocytosis, and washed extensively before destaining at 10 Hz for 120 seconds. n = 3 coverslips, 71 boutons for αsyn-transfected, 73 nerve terminals for vector. αsyn over-expression reduces the amount of releasable dye uptake (inset). p < 0.05, n = 6 coverslips, 153 boutons for αsyn-over-expressing cells, and 208 boutons for vector control from 2 independent transfections. (D) Hippocampal neurons transfected with VGLUT1-pHluorin and either human αsyn or empty vector were stimulated at 10 Hz for 150 s in the presence of 1 μM bafilomycin to reveal the full recycling pool, followed by treatment with NH4Cl (arrow) to reveal the total synaptic vesicle pool. n = 60 boutons from 3 coverslips for each condition (E) Left panel - cultures were transfected and stimulated as in C but with controls stimulated in the presence of 1 mM as well as 2 mM Ca2+. n = 60 boutons from 3 coverslips per condition. Right panel - peak ΔF/F0 before addition of NH4Cl (normalized to 2 mM Ca2+ control) shows a reduction in the presence of over-expressed αsyn but no significant reduction of control boutons stimulated in 1 mM Ca2+. p<0.01 by one-way ANOVA with Tukey’s post hoc tests, p>0.05 for vector in 2 mM Ca2+ versus vector in 1 mM Ca2+, p<0.01 for vector in 2 mM Ca2+ versus αsyn in 2 mM Ca2+, and p<0.05 for vector in 1 mM Ca2+ versus αsyn in 2 mM Ca2+. n=120 boutons from 6 coverslips per condition and 2 independent transfections. Values represent mean ± SEM.
Figure 5. The N-terminus, but not the C-terminus, of α-synuclein is required for the inhibition of synaptic vesicle exocytosis
(A) Time course of VGLUT1-pHluorin fluorescence during a 10 Hz stimulus for 60 seconds in neurons expressing either vector, αsyn, or the PD-associated mutant A30P. (B) Peak ΔF/F0 normalized to the response in vector control shows that the A30P mutation abolishes the inhibition of neurotransmitter release by αsyn, but the A53T and E46K mutations have no effect. p<0.0001 by one-way ANOVA. p<0.001 for A30P versus wild type by Tukey’s multiple comparison test. n = 180 boutons from 9 coverslips per condition and 3 independent transfections. (C) Neurons transfected with wild type αsyn and the C-terminal truncation 1–110 also show a similar inhibition of synaptic vesicle exocytosis relative to vector control. (D) Peak ΔF/F0 normalized to the response in vector control shows that deletion of the C-terminus has no effect on the inhibition of neurotransmitter release by αsyn. The closely related isoform βsyn inhibits neurotransmitter release to a similar extent. p<0.01 by one-way ANOVA, but p>0.05 for 1–110 and βsyn versus wild type by Tukey’s multiple comparison test. n = 120 boutons from 6 coverslips per condition and 2 independent transfections. Values represent mean ± SEM. (E) Time course of changes in the fluorescence of VGLUT1-pHluorin during and after 10 Hz stimulation for 60 seconds in neurons transfected with 1.5 μg VGLUT1-pHluorin and either 1.5 μg vector control (vec), 1.5 μg αsyn, or 1.0 μg vector and 0.5 μg αsyn (0.5 μg αsyn). n = 3 coverslips, 60 boutons per condition. (F) Peak ΔF/F0 values at the end of the stimulus. Values are normalized to the response in the vector control condition. n=3 coverslips, 60 boutons per condition. The bars indicate mean ± SEM.
Figure 6. α –Synuclein causes a selective decrease in the level of synapsins and complexins
Western analysis of brain extracts from αsyn transgenic mice and wildtype littermates using fluorescent secondary antibodies show a reduction in synapsins (A) and complexins (B). (C) Quantitation shows no change in the level of many other synaptic proteins. n = 3 animals per genotype. Values represent mean ± SEM.
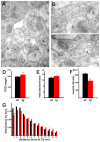
Figure 7. Over-expression of α-synuclein disrupts synaptic vesicle clustering
(A) An electron micrograph from stratum radiatum of hippocampal region CA1 in a wild type mouse shows clustered vesicles (*) in a terminal (T) closely apposed to the post-synaptic density of a dendritic spine (S). Although the nerve terminal and distal axons (A) are relatively large structures, their synaptic vesicles cluster (*), leaving most of the axoplasm unoccupied. (B) In a representative sex-matched transgenic sibling, synaptic vesicles show less clustering at the active zone, and occupy the full volume of the distal axon (*). (C) The distal axon of a transgenic mouse shows synaptic vesicles dispersed into the axon. Scale bar: 100 nm (A–C). (D) The post-synaptic density (PSD) is slightly longer in transgenic mice (p<0.05, n=217 for wild type and 199 for transgenic mice). Quantitation of synaptic vesicle number associated with a particular active zone (E) shows no significant differences between wild type and transgenic mice (p=0.37, n=199, 218). (F) In contrast, synaptic vesicle density in synaptic boutons of transgenic mice shows a substantial reduction relative to wild type (p<0.0001, n=217 for wild type and 201 for transgenic mice). (G) Histogram with bins indicating the following distances from the active zone: 1:25–50 nm, 2:50–75 nm, 3:75–100 nm, 4:100–125 nm, 5:125–150 nm, 6:150–175 nm, 7:175–200 nm, 8:200–225 nm, 9:225–250 nm, 10:250–275 nm, 11:275–300 nm, 12:300–325 nm, 13:325–350 nm. n = 2714 vesicles for wild type and 3193 for transgenic. A chi-square test shows p<0.05 for bins marked with * and p<0.0001 for bins marked with ***.
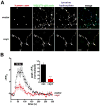
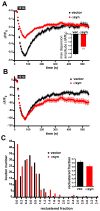
Figure 8. Over-expression of α-synuclein inhibits synaptic vesicle reclustering after endocytosis
(A) Time course of the synaptic GFP-VGLUT1 response to 10 Hz, 60 s stimulation in rat hippocampal neurons transfected with either human αsyn or vector control. Inset shows the amplitude of dispersion measured at the end of the electrical stimulus. ***, p<0.0001 by unpaired, two-tailed t-test. n=131 synapses for vector control and n=135 synapses from αsyn-transfected cells, 9 coverslips each, from 3 independent transfections. (B) Normalization of the data from (A) to the maximum extent of dispersion shows that boutons over-expressing αsyn exhibit a defect in the extent of reclustering. Inset shows, however, that there is no change in the time constant τ of reclustering. n=152 synapses for vector control and n=172 synapses from αsyn-transfected cells, 9 coverslips each from 3 independent transfections. (C) Distribution of boutons by the extent of reclustering shows that αsyn increases the proportion with reduced reclustering. p < 0.0001 by chi-square analysis. Inset shows the average reclustered fraction. n=154 synapses for vector control and n=168 synapses from αsyn-transfected cells, 9 coverslips each, from 3 independent transfections.
Comment in
- alpha-Synuclein at the synaptic gate.
Surmeier DJ. Surmeier DJ. Neuron. 2010 Jan 14;65(1):3-4. doi: 10.1016/j.neuron.2009.12.030. Neuron. 2010. PMID: 20152107
Similar articles
- Huntingtin-associated protein-1 is a synapsin I-binding protein regulating synaptic vesicle exocytosis and synapsin I trafficking.
Mackenzie KD, Lumsden AL, Guo F, Duffield MD, Chataway T, Lim Y, Zhou XF, Keating DJ. Mackenzie KD, et al. J Neurochem. 2016 Sep;138(5):710-21. doi: 10.1111/jnc.13703. Epub 2016 Jul 18. J Neurochem. 2016. PMID: 27315547 - Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis.
Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. Larsen KE, et al. J Neurosci. 2006 Nov 15;26(46):11915-22. doi: 10.1523/JNEUROSCI.3821-06.2006. J Neurosci. 2006. PMID: 17108165 Free PMC article. - Inhibition of exocytosis or endocytosis blocks activity-dependent redistribution of synapsin.
Orenbuch A, Shulman Y, Lipstein N, Bechar A, Lavy Y, Brumer E, Vasileva M, Kahn J, Barki-Harrington L, Kuner T, Gitler D. Orenbuch A, et al. J Neurochem. 2012 Jan;120(2):248-58. doi: 10.1111/j.1471-4159.2011.07579.x. Epub 2011 Dec 2. J Neurochem. 2012. PMID: 22066784 - The physiological role of α-synuclein and its relationship to Parkinson's Disease.
Sulzer D, Edwards RH. Sulzer D, et al. J Neurochem. 2019 Sep;150(5):475-486. doi: 10.1111/jnc.14810. Epub 2019 Jul 28. J Neurochem. 2019. PMID: 31269263 Free PMC article. Review. - The synaptic vesicle cycle.
Sudhof TC. Sudhof TC. Annu Rev Neurosci. 2004;27:509-47. doi: 10.1146/annurev.neuro.26.041002.131412. Annu Rev Neurosci. 2004. PMID: 15217342 Review.
Cited by
- Effect of amyloids on the vesicular machinery: implications for somatic neurotransmission.
Das AK, Pandit R, Maiti S. Das AK, et al. Philos Trans R Soc Lond B Biol Sci. 2015 Jul 5;370(1672):20140187. doi: 10.1098/rstb.2014.0187. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26009766 Free PMC article. Review. - Did α-Synuclein and Glucocerebrosidase Coevolve? Implications for Parkinson's Disease.
Gruschus JM. Gruschus JM. PLoS One. 2015 Jul 27;10(7):e0133863. doi: 10.1371/journal.pone.0133863. eCollection 2015. PLoS One. 2015. PMID: 26214314 Free PMC article. - Tau knockout exacerbates degeneration of parvalbumin-positive neurons in substantia nigra pars reticulata in Parkinson's disease-related α-synuclein A53T mice.
Jiao L, Zheng M, Duan J, Wu T, Li Z, Liu L, Xiang X, Tang X, He J, Li X, Zhang G, Ding J, Cai H, Lin X. Jiao L, et al. FASEB J. 2020 Sep;34(9):12239-12254. doi: 10.1096/fj.202000017RR. Epub 2020 Jul 30. FASEB J. 2020. PMID: 33000527 Free PMC article. - Hunting for Genes Underlying Emotionality in the Laboratory Rat: Maps, Tools and Traps.
Ramos A, Granzotto N, Kremer R, Boeder AM, de Araújo JFP, Pereira AG, Izídio GS. Ramos A, et al. Curr Neuropharmacol. 2023;21(9):1840-1863. doi: 10.2174/1570159X20666220901154034. Curr Neuropharmacol. 2023. PMID: 36056863 Free PMC article. Review. - Characterization of cognitive deficits in rats overexpressing human alpha-synuclein in the ventral tegmental area and medial septum using recombinant adeno-associated viral vectors.
Hall H, Jewett M, Landeck N, Nilsson N, Schagerlöf U, Leanza G, Kirik D. Hall H, et al. PLoS One. 2013 May 21;8(5):e64844. doi: 10.1371/journal.pone.0064844. Print 2013. PLoS One. 2013. PMID: 23705016 Free PMC article.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
- Banker G, Goslin K. Culturing nerve cells. 2. Cambridge, MA: MIT Press; 1998.
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. - PubMed
- Bussell R, Jr, Eliezer D. A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–778. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases