Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza - PubMed (original) (raw)
Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza
Robert E Ariano et al. CMAJ. 2010.
Abstract
Background: Whether the enteric absorption of the neuraminidase inhibitor oseltamivir is impaired in critically ill patients is unknown. We documented the pharmacokinetic profile of oseltamivir in patients admitted to intensive care units (ICUs) with suspected or confirmed pandemic (H1N1) influenza.
Methods: We included 41 patients 18 years of age and older with suspected or confirmed pandemic (H1N1) influenza who were admitted for ventilatory support to nine ICUs in three cities in Canada and Spain. Using tandem mass spectrometry, we assessed plasma levels of oseltamivir free base and its active metabolite carboxylate at baseline (before gastric administration of the drug) and at 2, 4, 6, 9 and 12 hours after the fourth or later dose.
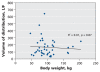
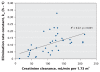
Results: Among the 36 patients who did not require dialysis, the median concentration of oseltamivir free base was 10.4 (interquartile range [IQR] 4.8-14.9) microg/L; the median concentration of the carboxylate metabolite was 404 (IQR 257-900) microg/L. The volume of distribution of the carboxylate metabolite did not increase with increasing body weight (R2=0.00, p=0.87). The rate of elimination of oseltamivir carboxylate was modestly correlated with estimations of creatinine clearance (R2=0.27, p<0.001). Drug clearance in the five patients who required continuous renal replacement therapy was about one-sixth that in the 36 patients with relatively normal renal function.
Interpretation: Oseltamivir was well absorbed enterically in critically ill patients admitted to the ICU with suspected or confirmed pandemic (H1N1) influenza. The dosage of 75 mg twice daily achieved plasma levels that were comparable to those in ambulatory patients and were far in excess of concentrations required to maximally inhibit neuraminidase activity of the virus. Adjustment of the dosage in patients with renal dysfunction requiring continuous renal replacement therapy is appropriate; adjustment for obesity does not appear to be necessary.
Figures
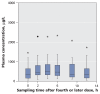
Figure 1
Average plasma concentrations of oseltamivir carboxylate over one dosing interval in 34 patients admitted to intensive care unit (ICU) with suspected or confirmed pandemic (H1N1) influenza who did not require dialysis (primary study cohort). Oseltamivir doses were normalized to 75 mg twice daily. The horizontal line in the middle of each box indicates the median, and the top and bottom borders of the box mark the 25th and 75th percentiles, respectively. The whiskers are 1.5 times the upper and lower interquartile ranges. The outlier (black dot) indicates a case with values between 1.5 and 3 box lengths from one side of the range. The extreme values (*) indicate cases with values more than 3 box lengths from the 75th or 25th percentile.
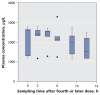
Figure 2
Average plasma concentrations of oseltamivir carboxylate over one dosing interval in five patients admitted to ICU with suspected or confirmed pandemic (H1N1) influenza who received continuous renal replacement therapy. Oseltamivir doses were normalized to 75 mg twice daily. The horizontal line in the middle of each box indicates the median, and the top and bottom borders of the box mark the 25th and 75th percentiles, respectively. The whiskers are 1.5 times the upper and lower interquar-tile ranges. The outlier (black dot) indicates a case with values between 1.5 and 3 box lengths from one side of the range.
Figure 3
Scatterplot showing the relation between body weight and volume of distribution for oseltamivir carboxylate. Solid line represents the linear regression fit across all patients. F = systemic bioavailability.
Figure 4
Scatterplot showing the relation between normalized creatinine clearance and the elimination rate of oseltamivir carboxylate. Solid line represents the linear regression fit across all patients.
Similar articles
- Oseltamivir pharmacokinetics in critically ill adults receiving extracorporeal membrane oxygenation support.
Mulla H, Peek GJ, Harvey C, Westrope C, Kidy Z, Ramaiah R. Mulla H, et al. Anaesth Intensive Care. 2013 Jan;41(1):66-73. doi: 10.1177/0310057X1304100112. Anaesth Intensive Care. 2013. PMID: 23362894 Clinical Trial. - Oseltamivir in seasonal, avian H5N1 and pandemic 2009 A/H1N1 influenza: pharmacokinetic and pharmacodynamic characteristics.
Widmer N, Meylan P, Ivanyuk A, Aouri M, Decosterd LA, Buclin T. Widmer N, et al. Clin Pharmacokinet. 2010 Nov;49(11):741-65. doi: 10.2165/11534730-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20923248 Review. - Impact of extracorporeal membrane oxygenation and continuous venovenous hemodiafiltration on the pharmacokinetics of oseltamivir carboxylate in critically ill patients with pandemic (H1N1) influenza.
Lemaitre F, Luyt CE, Roullet-Renoleau F, Nieszkowska A, Zahr N, Corvol E, Fernandez C, Antignac M, Farinotti R, Combes A. Lemaitre F, et al. Ther Drug Monit. 2012 Apr;34(2):171-5. doi: 10.1097/FTD.0b013e318248672c. Ther Drug Monit. 2012. PMID: 22354159 - Pharmacokinetics of oseltamivir and oseltamivir carboxylate in critically ill patients receiving continuous venovenous hemodialysis and/or extracorporeal membrane oxygenation.
Eyler RF, Heung M, Pleva M, Sowinski KM, Park PK, Napolitano LM, Mueller BA. Eyler RF, et al. Pharmacotherapy. 2012 Dec;32(12):1061-9. doi: 10.1002/phar.1151. Pharmacotherapy. 2012. PMID: 23208833 - How to manage antivirals in critically ill patients with influenza?
Bay P, Martin-Loeches I, Haudebourg AF, Lê MP, Peytavin G, Rameix-Welti MA, Fourati S, de Prost N. Bay P, et al. Clin Microbiol Infect. 2025 Jul;31(7):1157-1165. doi: 10.1016/j.cmi.2025.04.002. Epub 2025 Apr 7. Clin Microbiol Infect. 2025. PMID: 40204233 Review.
Cited by
- Severe community-acquired pneumonia.
Sligl WI, Marrie TJ. Sligl WI, et al. Crit Care Clin. 2013 Jul;29(3):563-601. doi: 10.1016/j.ccc.2013.03.009. Crit Care Clin. 2013. PMID: 23830654 Free PMC article. Review. - JAID/JSC Guidelines for the Treatment of Respiratory Infectious Diseases: The Japanese Association for Infectious Diseases/Japanese Society of Chemotherapy - The JAID/JSC Guide to Clinical Management of Infectious Disease/Guideline-preparing Committee Respiratory Infectious Disease WG.
Mikasa K, Aoki N, Aoki Y, Abe S, Iwata S, Ouchi K, Kasahara K, Kadota J, Kishida N, Kobayashi O, Sakata H, Seki M, Tsukada H, Tokue Y, Nakamura-Uchiyama F, Higa F, Maeda K, Yanagihara K, Yoshida K. Mikasa K, et al. J Infect Chemother. 2016 Jul;22(7 Suppl):S1-S65. doi: 10.1016/j.jiac.2015.12.019. Epub 2016 Jun 15. J Infect Chemother. 2016. PMID: 27317161 Free PMC article. No abstract available. - Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution.
Honce R, Schultz-Cherry S. Honce R, et al. Front Immunol. 2019 May 10;10:1071. doi: 10.3389/fimmu.2019.01071. eCollection 2019. Front Immunol. 2019. PMID: 31134099 Free PMC article. Review. - Early emergence of an H275Y mutation in a hematopoietic cell transplant recipient treated with intravenous peramivir.
Renaud C, Pergam SA, Polyak C, Jain R, Kuypers J, Englund JA, Corey L, Boeckh MJ. Renaud C, et al. Transpl Infect Dis. 2010 Dec;12(6):513-7. doi: 10.1111/j.1399-3062.2010.00582.x. Epub 2010 Nov 10. Transpl Infect Dis. 2010. PMID: 21062390 Free PMC article. - Pharmacokinetics of oseltamivir carboxylate in critically ill patients: each patient is unique.
Kromdijk W, Sikma MA, van den Broek MP, Beijnen JH, Huitema AD, de Lange DW. Kromdijk W, et al. Intensive Care Med. 2013 May;39(5):977-8. doi: 10.1007/s00134-013-2851-x. Epub 2013 Feb 27. Intensive Care Med. 2013. PMID: 23443310 Free PMC article. No abstract available.
References
- Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–9. - PubMed
- Dominguez-Cherit G, Lapinsky SE, Espinosa-Perez L, et al. Critically ill patients with influenza A (novel H1N1) in Mexico. JAMA. 2009;302:1880–7. - PubMed
- World Health Organization. WHO guidelines for pharmacologic management of pandemic (H1N1) 2009 influenza and other influenza viruses. Geneva (Switzerland): The Organization; 2009.
- World Health Organization. Clinical management of human infection with pandemic (H1N1) 2009: revised guidance. Geneva (Switzerland): The Organization; 2009.
- Power BM, Forbes AM, van Heerden PV, et al. Pharmacokinetics of drugs used in critically ill adults. Clin Pharmacokinet. 1998;34:25–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical