Avian magnetoreception: elaborate iron mineral containing dendrites in the upper beak seem to be a common feature of birds - PubMed (original) (raw)
Avian magnetoreception: elaborate iron mineral containing dendrites in the upper beak seem to be a common feature of birds
Gerald Falkenberg et al. PLoS One. 2010.
Abstract
The magnetic field sensors enabling birds to extract orientational information from the Earth's magnetic field have remained enigmatic. Our previously published results from homing pigeons have made us suggest that the iron containing sensory dendrites in the inner dermal lining of the upper beak are a candidate structure for such an avian magnetometer system. Here we show that similar structures occur in two species of migratory birds (garden warbler, Sylvia borin and European robin, Erithacus rubecula) and a non-migratory bird, the domestic chicken (Gallus gallus). In all these bird species, histological data have revealed dendrites of similar shape and size, all containing iron minerals within distinct subcellular compartments of nervous terminals of the median branch of the Nervus ophthalmicus. We also used microscopic X-ray absorption spectroscopy analyses to identify the involved iron minerals to be almost completely Fe III-oxides. Magnetite (Fe II/III) may also occur in these structures, but not as a major Fe constituent. Our data suggest that this complex dendritic system in the beak is a common feature of birds, and that it may form an essential sensory basis for the evolution of at least certain types of magnetic field guided behavior.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
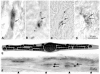
Figure 1. Prussian blue stained dendrites in the inner lining of the upper beak of various bird species.
(A–D) The dendrites have a similar light microscopic structure irrespective of the avian species (A homing pigeon, B garden warbler, C European robin, D domestic chicken). Over a length of about 20 to 30 µm several iron rich bullets (diameter 1 µm) can be found together with a centrally located vesicle (diameter about 5 µm, arrows point to vesicles). (Same scale in A–D; 10 µm paraffin sections, Prussian blue staining). (E) General semi-schematic drawing of an iron containing dendrite (after [19]). (F) Axon bundle with several iron containing dendrites. The dendrites are aligned in a distinct micro architecture: Parallel dendrites lie closely attached to each other (arrows). The dendritic groups keep a longitudinal regular distance (arrowheads). (Scale bar 20 µm; sagittal 10 µm thick paraffin section of a pigeon beak; stack reconstruction of different focal planes).
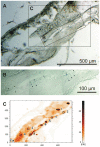
Figure 2. Identification of iron containing dendrites in stained and unstained neighboring sections of a garden warbler beak.
(A) A Prussian Blue (PB) stained section of the beak of a garden warbler serves as reference. (Scale bar 500 µm; 10 µm thick paraffin section). (B) Chain of dendrites identified by Prussian blue staining reconstructed from different focal planes. The major part of these dendrites lies in the unstained neighboring section and was there detected by SXRF (arrow heads point to the dendrites; same section as in A at a higher magnification, see frame B in A; scale bar 100 µm; 10 µm Paraffin section). (C) Microscopic SXRF map of Fe of an unstained section neighboring the PB-stained section in A (see frame C in A; section mounted on Ultralene foil). The element map of iron shows a typical accumulation of iron at sites matching PB stained dendrites in A/B. (Squares with numbers indicate the measuring points for the subsequent µ-XANES analyses; same scale as in A).
Figure 3. Qualitative distribution of various elements determined by µ-XRF analysis in an unstained section of a warbler beak.
According to tissue specificity (see schematic drawing lower right corner), element maps show characteristic differences, which help to further differentiate between the various iron containing structures. For example, dendritic Fe occurs clearly aligned along a delicate structure, an axon bundle, with Ca in a higher concentration compared to the direct vicinity. (The symbols with numbers correspond to the XANES spectra shown in Fig. 5)
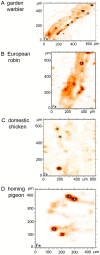
Figure 4. Micro-SXRF Fe-maps of dendritic areas in four avian species.
According to the criteria described by Figure 2, areas with iron containing dendrites are selected for micro-SXRF maps in different bird species (A) garden warbler, (B) European robin, (C) domestic chicken, (D) homing pigeon. Here the Fe-maps are shown, only. (10 µm sections mounted on Ultralene foil). The measuring sites in these examples of beak sections for the subsequent micro-XANES analyses (see Figure 6) are marked.
Figure 5. µ-XANES measurements at different sites in an unstained section of the beak of a garden warbler.
The spectra measured at the putative dendritic sites (see Figure 3: pt 2, pt 4–9) are very similar, while those of the contamination (pt 1, pt 3) are clearly different and shifted to lower energies. Small deviations of the spectra must be attributed to statistics. (Pt = measuring site)
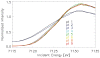
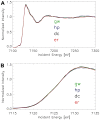
Figure 6. Micro-XANES-spectra of avian dendrites.
X-ray spectra at the K-edge of iron of different birds (normalized). The spectra are extremely similar over the complete XANES and extended XANES range. (A) XANES-spectra in the energy range between 7100 eV and 7300 eV incident energy, (B) The same spectra shown over the energy range between 7115 eV and 7135 eV. All studied avian tissues have spectra of the same edge position and shape. (Averaged and normalized data from several measurements; green line = Garden warbler, blue line = Homing pigeon, black line = Domestic chicken, red line = European robin.)
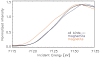
Figure 7. Summed micro-XANES spectra of biological (avian) tissue at dendrite positions compared to measured standard Fe compounds, magnetite and maghemite.
The spectrum of the dendritic bird tissue does not completely match with the spectra of any Fe oxides. The maghemite spectrum (blue line) approaches the birds' spectrum (stippled black line) in the lower energy range very closely, but shows a significant deviation at energy above 7126 eV. However, the edge of magnetite (orange line) is shifted to lower energies, indicating its different oxidation state. Hence, the iron inside the biological material cannot be solely composed of magnetite.
Similar articles
- A novel concept of Fe-mineral-based magnetoreception: histological and physicochemical data from the upper beak of homing pigeons.
Fleissner G, Stahl B, Thalau P, Falkenberg G, Fleissner G. Fleissner G, et al. Naturwissenschaften. 2007 Aug;94(8):631-42. doi: 10.1007/s00114-007-0236-0. Epub 2007 Mar 15. Naturwissenschaften. 2007. PMID: 17361399 - The magnetite-based receptors in the beak of birds and their role in avian navigation.
Wiltschko R, Wiltschko W. Wiltschko R, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013 Feb;199(2):89-98. doi: 10.1007/s00359-012-0769-3. Epub 2012 Oct 31. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013. PMID: 23111859 Free PMC article. Review. - Theoretical analysis of an iron mineral-based magnetoreceptor model in birds.
Solov'yov IA, Greiner W. Solov'yov IA, et al. Biophys J. 2007 Sep 1;93(5):1493-509. doi: 10.1529/biophysj.107.105098. Epub 2007 May 11. Biophys J. 2007. PMID: 17496012 Free PMC article. - Trigeminally innervated iron-containing structures in the beak of homing pigeons, and other birds.
Williams MN, Wild JM. Williams MN, et al. Brain Res. 2001 Jan 19;889(1-2):243-6. doi: 10.1016/s0006-8993(00)03114-0. Brain Res. 2001. PMID: 11166712 - [Magnetoreception systems in birds: a review of current research].
Kishkinev DA, Chernetsov NS. Kishkinev DA, et al. Zh Obshch Biol. 2014 Mar-Apr;75(2):104-23. Zh Obshch Biol. 2014. PMID: 25490840 Review. Russian.
Cited by
- In Search for the Avian Trigeminal Magnetic Sensor: Distribution of Peripheral and Central Terminals of Ophthalmic Sensory Neurons in the Night-Migratory Eurasian Blackcap (Sylvia atricapilla).
Haase K, Musielak I, Warmuth-Moles L, Leberecht B, Zolotareva A, Mouritsen H, Heyers D. Haase K, et al. Front Neuroanat. 2022 Mar 7;16:853401. doi: 10.3389/fnana.2022.853401. eCollection 2022. Front Neuroanat. 2022. PMID: 35321391 Free PMC article. - Conditioning domestic chickens to a magnetic anomaly.
Denzau S, Kuriakose D, Freire R, Munro U, Wiltschko W. Denzau S, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2011 Dec;197(12):1137-41. doi: 10.1007/s00359-011-0675-0. Epub 2011 Sep 6. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2011. PMID: 21894488 - Migratory blackcaps can use their magnetic compass at 5 degrees inclination, but are completely random at 0 degrees inclination.
Schwarze S, Steenken F, Thiele N, Kobylkov D, Lefeldt N, Dreyer D, Schneider NL, Mouritsen H. Schwarze S, et al. Sci Rep. 2016 Sep 26;6:33805. doi: 10.1038/srep33805. Sci Rep. 2016. PMID: 27667569 Free PMC article. - Acuity of a cryptochrome and vision-based magnetoreception system in birds.
Solov'yov IA, Mouritsen H, Schulten K. Solov'yov IA, et al. Biophys J. 2010 Jul 7;99(1):40-9. doi: 10.1016/j.bpj.2010.03.053. Biophys J. 2010. PMID: 20655831 Free PMC article. - High resolution anatomical mapping confirms the absence of a magnetic sense system in the rostral upper beak of pigeons.
Treiber CD, Salzer M, Breuss M, Ushakova L, Lauwers M, Edelman N, Keays DA. Treiber CD, et al. Commun Integr Biol. 2013 Jul 1;6(4):e24859. doi: 10.4161/cib.24859. Epub 2013 May 10. Commun Integr Biol. 2013. PMID: 23940826 Free PMC article.
References
- Wiltschko W, Merkel FW. Zugorientierung van Dorngrasmucken (Sylvia communis) im Erdmagnetfeld. Vogelwarte. 1971;26:245–249.
- Wiltschko W, Wiltschko R. Magnetic compass of European robins. Science. 1972;176:62–64. - PubMed
- Cochran WW, Mouritsen H, Wikelski M. Migrating songbirds recalibrate their magnetic compass daily from twilight cues. Science. 2004;304(5669):405–408. - PubMed
- Johnsen S, Lohmann KJ. The physics and neurobiology of magnetoreception. Nature Rev Neuroscience. 2005;6:703–712. - PubMed
- Mouritsen H, Ritz T. Magnetoreception and its use in bird navigation. Curr Opinion Neurobiol. 2005;15:406–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical