Targeting the FANCJ-BRCA1 interaction promotes a switch from recombination to poleta-dependent bypass - PubMed (original) (raw)
. 2010 Apr 29;29(17):2499-508.
doi: 10.1038/onc.2010.18. Epub 2010 Feb 22.
Affiliations
- PMID: 20173781
- PMCID: PMC2909592
- DOI: 10.1038/onc.2010.18
Targeting the FANCJ-BRCA1 interaction promotes a switch from recombination to poleta-dependent bypass
J Xie et al. Oncogene. 2010.
Abstract
BRCA1 and the DNA helicase FANCJ (also known as BACH1 or BRIP1) have common functions in breast cancer suppression and DNA repair. However, the functional significance of the direct interaction between BRCA1 and FANCJ remains unclear. Here, we have discovered that BRCA1 binding to FANCJ regulates DNA damage repair choice. Thus, when FANCJ binding to BRCA1 is ablated, the molecular mechanism chosen for the repair of damaged DNA is dramatically altered. Specifically, a FANCJ protein that cannot be phosphorylated at serine 990 or bind BRCA1 inhibits DNA repair via homologous recombination and promotes poleta-dependent bypass. Furthermore, the poleta-dependent bypass promoted by FANCJ requires the direct binding to the mismatch repair (MMR) protein, MLH1. Together, our findings implicate that in human cells BRCA1 binding to FANCJ is critical to regulate DNA repair choice and promote genomic stability. Moreover, unregulated FANCJ function could be associated with cancer and/or chemoresistance.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures
Figure 1
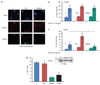
FANCJS990A, as compared with FANCJWT, promotes a distinct DNA damage response in FA-J cells. (a) FANCJ-null FA-J cells were complemented with vector, FANCJWT, or FANCJS990A and lysates were analyzed by immunoblot with the indicated antibodies (Abs). (b) The FA-J cell lines were treated constitutively with 0.25 µg/ml melphalan, collected at the indicated times, and analyzed by FACS to determine the percentage of cells in G2/M. A representative experiment is shown. The bar graph represents mean ± s.d. from three independent experiments. The asterisk indicates a significant difference (P<0.05, unpaired _t_-test). (c) The FA-J cells expressing vector, FANCJWT, or FANCJS990A were plated at low density, treated with the indicated doses of cisplatin, UV, zeocin, 6-thioguanine, or methyl methanesulfonate and allowed to grow for 5–8 days. The cells were then collected and counted to analyze percent growth. Data represent mean percent ± s.d. of growth from three independent experiments.
Figure 2
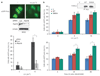
FANCJS990A reduces DSB-induced RAD51 foci and HR. (a) The FA-J cell lines were treated with 12.5 µg/ml of zeocin and immunofluorescence was performed with γ-H2AX and RAD51 Abs. A representative image is shown to depict how staining was observed. (b) The γ-H2AX and (c) RAD51 foci were quantitated based on a cell being positive (> 10) foci per 300 DAPI positive cells from three independent experiments. Asterisk indicates significant difference (P<0.05, unpaired _t_-test). (d) DR-U2OS cells were cotransfected with the I-Sce-1 endonuclease and vector, FANCJWT, FANCJS990A, or FANCJK52R, collected and either lysed and immunoprecipitated followed by immunoblot with the indicated Abs or analyzed by FACS. The bar graph shows the percentage of GFP-positive cells.
Figure 3
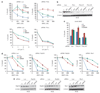
FANCJ enhances DNA damage-induced polη foci. (a) U2OS cells were cotransfected with siRNA for luc or Rad18 and eGFP-polη and either collected for immunoblot with the indicated Abs or UV irradiated and assessed for eGFP-polη foci. Cells were assessed for eGFP-polη foci by autofluorescence. (b) U2OS cells stably expressing vector, FANCJS990A, or FANCJWT were transfected with eGFP-polη and either collected for immunoblot with the indicated Abs or UV irradiated with indicated dose with 4 h incubation or treated with 250 n
m
MMC with incubation at varying times. Data represent the mean percent ± s.d. cells positive (> 10) green foci per 300 DAPI positive cells from three independent experiments. Asterisk indicates significant difference (P<0.05, unpaired _t_-test).
Figure 4
FANCJS990A promotes ICL and UV resistance in a polη-dependent manner. (a) FA-J cells stably expressing vector, FANCJWT, or FANCJS990A (Figure 1a) were transfected with siRNA to Luc or polη, incubated for 48 h, treated with MMC or UV, and percent growth was assessed as in Figure 1. (b) FA-J cell lines transfected with siRNA to luc, polη, polη #1, or polη #2 were collected for immunoblot with the indicated Abs. (c) The FA-J cell lines with indicated siRNAs were incubated for 48 h, treated with MMC at the IC50 dose, and percent growth was assessed as in Figure 1. Graph shows the percent growth mean±s.d. (d) FA-J cells stably expressing vector, FANCJWT, or FANCJS990A were transfected with siRNA to luc, Rad18, Rad54, or Rev1 and incubated for 48 h, treated with MMC, and percent growth was assessed as in Figure 1. (e) Cells used in (d) were collected and lysed for immunoblot with the indicated Abs.
Figure 5
FANCJS990A requires MLH1 binding to promote polη-dependent bypass. (a) FA-J cells stably expressing vector, FANCJWT, FANCJS990A, or FANCJK141/142A were collected and immunoprecipitated with anti-FANCJ Abs and blotted with the indicated Abs. (b) FA-J cells stably expressing vector, FANCJWT, FANCJS990A, FANCJK141/142A, or FANCJK141/142A/S990A were either collected for immunoblot with the indicated Abs or (c) treated with the indicated doses of MMC and allowed to grow for 5–8 days. The cells were then collected and counted to analyze percent growth. Data represent mean percent ± s.d. of growth from three independent experiments. (d) The FA-J cell lines were treated constitutively with 0.25 µg/ml melphalan, collected at the indicated times, and analyzed by FACS to determine the percentage of cells in G2/M. A representative experiment is shown. The bar graph represents mean ± s.d. from three independent experiments. (e) Model summarizes observations of this study. FANCJ when uncoupled from BRCA1 promotes polη-dependent TLS in a manner that requires MLH1 binding. Dotted line to MMR is added as a discussion point. To promote TLS, FANCJ could limit negative regulators of TLS, such as MMR.
Similar articles
- Assessing the link between BACH1/FANCJ and MLH1 in DNA crosslink repair.
Cantor SB, Xie J. Cantor SB, et al. Environ Mol Mutagen. 2010 Jul;51(6):500-7. doi: 10.1002/em.20568. Environ Mol Mutagen. 2010. PMID: 20658644 Review. - The FANCJ/MutLalpha interaction is required for correction of the cross-link response in FA-J cells.
Peng M, Litman R, Xie J, Sharma S, Brosh RM Jr, Cantor SB. Peng M, et al. EMBO J. 2007 Jul 11;26(13):3238-49. doi: 10.1038/sj.emboj.7601754. Epub 2007 Jun 21. EMBO J. 2007. PMID: 17581638 Free PMC article. - Crosstalk between BRCA-Fanconi anemia and mismatch repair pathways prevents MSH2-dependent aberrant DNA damage responses.
Peng M, Xie J, Ucher A, Stavnezer J, Cantor SB. Peng M, et al. EMBO J. 2014 Aug 1;33(15):1698-712. doi: 10.15252/embj.201387530. Epub 2014 Jun 25. EMBO J. 2014. PMID: 24966277 Free PMC article. - BRCA1-mediated repression of mutagenic end-joining of DNA double-strand breaks requires complex formation with BACH1.
Dohrn L, Salles D, Siehler SY, Kaufmann J, Wiesmüller L. Dohrn L, et al. Biochem J. 2012 Feb 1;441(3):919-26. doi: 10.1042/BJ20110314. Biochem J. 2012. PMID: 22032289 - Hereditary breast cancer and the BRCA1-associated FANCJ/BACH1/BRIP1.
Cantor SB, Guillemette S. Cantor SB, et al. Future Oncol. 2011 Feb;7(2):253-61. doi: 10.2217/fon.10.191. Future Oncol. 2011. PMID: 21345144 Free PMC article. Review.
Cited by
- Systematic investigation of BRCA1-A, -B, and -C complexes and their functions in DNA damage response and DNA repair.
Li S, Tang M, Xiong Y, Feng X, Wang C, Nie L, Huang M, Zhang H, Yin L, Zhu D, Yang C, Ma T, Chen J. Li S, et al. Oncogene. 2024 Aug;43(35):2621-2634. doi: 10.1038/s41388-024-03108-y. Epub 2024 Jul 27. Oncogene. 2024. PMID: 39068216 - The Adaptive Mechanisms and Checkpoint Responses to a Stressed DNA Replication Fork.
Saldanha J, Rageul J, Patel JA, Kim H. Saldanha J, et al. Int J Mol Sci. 2023 Jun 22;24(13):10488. doi: 10.3390/ijms241310488. Int J Mol Sci. 2023. PMID: 37445667 Free PMC article. Review. - Inhibitors of the Fanconi anaemia pathway as potential antitumour agents for ovarian cancer.
Taylor SJ, Arends MJ, Langdon SP. Taylor SJ, et al. Explor Target Antitumor Ther. 2020;1(1):26-52. doi: 10.37349/etat.2020.00003. Epub 2020 Feb 29. Explor Target Antitumor Ther. 2020. PMID: 36046263 Free PMC article. Review. - The repair gene BACH1 - a potential oncogene.
Muhseena N K, Mathukkada S, Das SP, Laha S. Muhseena N K, et al. Oncol Rev. 2021 Jul 2;15(1):519. doi: 10.4081/oncol.2021.519. eCollection 2021 Feb 26. Oncol Rev. 2021. PMID: 34322202 Free PMC article. - Comprehensive Mutational Analysis of the BRCA1-Associated DNA Helicase and Tumor-Suppressor FANCJ/BACH1/BRIP1.
Calvo JA, Fritchman B, Hernandez D, Persky NS, Johannessen CM, Piccioni F, Kelch BA, Cantor SB. Calvo JA, et al. Mol Cancer Res. 2021 Jun;19(6):1015-1025. doi: 10.1158/1541-7786.MCR-20-0828. Epub 2021 Feb 22. Mol Cancer Res. 2021. PMID: 33619228 Free PMC article.
References
- Albertella MR, Green CM, Lehmann AR, O’Connor MJ. A role for polymerase eta in the cellular tolerance to cisplatin-induced damage. Cancer Res. 2005;65:9799–9806. - PubMed
- Alt A, Lammens K, Chiocchini C, Lammens A, Pieck JC, Kuch D, et al. Bypass of DNA lesions generated during anticancer treatment with cisplatin by DNA polymerase eta. Science. 2007;318:967–970. - PubMed
- Barbour L, Xiao W. Regulation of alternative replication bypass pathways at stalled replication forks and its effects on genome stability: a yeast model. Mutat Res. 2003;532:137–155. - PubMed
- Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous