Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner - PubMed (original) (raw)
. 2010 May 5;11(5):390-401.
doi: 10.1016/j.cmet.2010.03.014.
Anand Selvaraj, So Young Kim, Pawan Gulati, Sophie Brûlé, Benoit Viollet, Bruce E Kemp, Nabeel Bardeesy, Patrick Dennis, John J Schlager, André Marette, Sara C Kozma, George Thomas
Affiliations
- PMID: 20444419
- PMCID: PMC3081779
- DOI: 10.1016/j.cmet.2010.03.014
Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner
Adem Kalender et al. Cell Metab. 2010.
Abstract
Dysfunctional mTORC1 signaling is associated with a number of human pathologies owing to its central role in controlling cell growth, proliferation, and metabolism. Regulation of mTORC1 is achieved by the integration of multiple inputs, including those of mitogens, nutrients, and energy. It is thought that agents that increase the cellular AMP/ATP ratio, such as the antidiabetic biguanides metformin and phenformin, inhibit mTORC1 through AMPK activation of TSC1/2-dependent or -independent mechanisms. Unexpectedly, we found that biguanides inhibit mTORC1 signaling, not only in the absence of TSC1/2 but also in the absence of AMPK. Consistent with these observations, in two distinct preclinical models of cancer and diabetes, metformin acts to suppress mTORC1 signaling in an AMPK-independent manner. We found that the ability of biguanides to inhibit mTORC1 activation and signaling is, instead, dependent on the Rag GTPases.
Figures
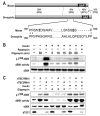
Figure 1. Oligomycin-induced dS6K dephosphorylation does not require dTSC1/2
(A) Alignment of Drosophila TSC2 (accession number NP_524177) with the mouse TSC2, isoform 1 (accession number NP_035777). Percentage of sequence identity and similarity (parentheses) of different regions of TSC2 are indicated. The major AMPK phosphorylation sites in TSC2 reported to be affected by energy depletion are in bold and underlined. The mouse 1271 and 1366 phosphorylation sites correspond to the respective rat 1227 and 1345 sites described by Inoki et al. GAP denotes the GTPase-activating domain (B) Analysis of dS6K activity following treatment of Kc167 cells with 10μM oligomycin for 30min or 100nM insulin for 30min. In samples containing 100nM insulin plus 10μM oligomycin, the inhibitor was added for the indicated time prior to the 30min insulin treatment. 20nM rapamycin was added 15min prior to 30min insulin treatment. Western blot analysis of dS6K T398 phosphorylation and dS6K are as indicated (C) Analysis of dS6K activity following dTsc1, dTsc2 dsRNA treatment in the absence or presence of either 100nM insulin or 10μM oligomycin for 30min. Western blot analysis of dS6K T398 phosphorylation, dS6K activity, dS6K and dTSC1 are as indicated. The data shown are representative of two independent experiments.
Figure 2. TSC2 is not required for downregulation of S6K1 T389 by energy-depleting agents
(A) TSC2+/+ or _TSC2_-/- MEFs were treated with either 10μM oligomycin or (B) 100mM 2DG for the times indicated. (C) TSC2+/+ or _TSC2_-/- MEFs were treated with the indicated concentrations of 2DG for 30min. (D) Western blot analysis of the indicated proteins in TSC2+/+ and _TSC2_-/- MEFs. (E and F) TSC2+/+ MEFs were treated with either 10μM oligomycin or 25mM 2DG at the indicated times. * denotes the addition of a fresh aliquot of either ATP-depleting agent following a 60min incubation with the initial inhibitor. The data shown are representative of at least three independent experiments.
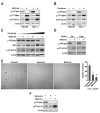
Figure 3. Metformin and phenformin inhibit mTORC1 signaling independent of TSC2
TSC2+/+ or _TSC2_-/- MEFs were treated with either (A) 10mM metformin for 24h or (B) 6mM phenformin for 1h. (C) _TSC2_-/- MEFs seeded at increasing density (1.25 ×104, 5 ×104, or 20 ×104 cells/well) were treated as in A. (D) _TSC2_-/- MEFs seeded at 20 ×104 cells/well and treated with 10mM metformin for 24h in either the initial or fresh medium. (E) TSC2+/-ang1 cells were allowed to grow in soft agar for 3 weeks, with or without 20nM rapamycin or 10mM metformin. Colonies were stained with MTT and counted as described in Experimental Procedures. (F) TSC2+/-ang1 cells were treated with either 20nM rapamycin for 15min or 10mM metformin for 24h. Western blots were performed as described in Experimental Procedures and probed with the indicated antibodies. The data shown are representative of at least two independent experiments.
Figure 4. AMPK is sufficient to inhibit mTORC1 signaling but it is not required for mTORC1 inhibition by energy depletion
(A) WT indicates HEK293 cells in which α, β, and γ subunits of AMPK were co-expressed for 40h. R70Q and R172Q indicate that the WT γ subunit was substituted with the respective γ mutants. After 24h of ectopic expression, cells were deprived of serum overnight and stimulated with 200nM insulin for 30min. C2C12 myoblasts were treated with either (B) 2mM AICAR or (C) 25mM 2DG for the indicated times. (D) Primary AMPK α1 and α2 double knock-out (AMPK dKO) and AMPK WT MEFS were treated with 2DG for 30min at the indicated concentrations. (E) Immortalized AMPK dKO and AMPK WT cells were starved of glucose for 1h. Western blots were performed as described in Experimental Procedures and using the indicated antibodies. The data shown are representative of at least two independent experiments
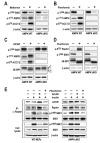
Figure 5. Metformin and phenformin, but not AICAR, inhibit mTORC1 independent of AMPK
Western blot analysis of extracts from AMPK dKO or AMPK WT MEFs treated either with (A) 10mM metformin for 24h, (B and D) 6mM phenformin for 1h, or (C) 2mM AICAR for 2h. (E) Serum-starved AMPK dKO or AMPK WT MEFs treated for 1h with phenformin or AICAR were stimulated with insulin for 15 min. Raptor was immunoprecipitated, and in vitro mTORC1 activity was assayed as described previously (Gulati et al., 2008; Sancak et al., 2007). In parallel, western blot analyses were performed on cell lysates. The data shown are representative of multiple independent experiments.
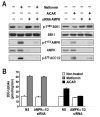
Figure 6. Absence of AMPK does not affect metformin-mediated glucose uptake
(A) L6 myotubes were transfected with either non-silencing dsRNA (NS) or with two different dsRNAs directed against either the α1 or the α2 catalytic subunit of AMPK and incubated for 72 hrs. Cells were then treated with either 5mM metformin for the last 24 hrs or 2mM AICAR for the last 2 hrs. Parallel plates were either extracted for western blotting with the indicated antibodies or (B) further processed for glucose-uptake measurements, as described in Experimental Procedures. Results are representative of three independent experiments conducted in triplicate.
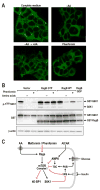
Figure 7. Rag GTPases mediate the inhibition of mTORC1 by Phenformin
(A) HEK293T cells were either starved for serum and AAs for 50 min (-AA) and re-stimulated with AAs for 10min (-AA→+AA), or cells in full media treated with 5mM phenformin for 1hr. Cells were processed as described in Experimental Procedures and immunofluorescence assays were performed to detect mTOR (green) (B) HEK293T cells with PRK5 empty vector, used as a transfection control, or expressing epitope-tagged HA-GST-RagB GTP or WT HA- GST-RagB were deprived of AAs for 1hr, or treated with 5mM phenformin for 1hr. Western blots were performed as described in Experimental Procedures and proteins were detected with the indicated antibodies. These studies are representative of at least two independent experiments (C) Model for metformin/phenformin action on mTORC1.
Similar articles
- cAMP inhibits mammalian target of rapamycin complex-1 and -2 (mTORC1 and 2) by promoting complex dissociation and inhibiting mTOR kinase activity.
Xie J, Ponuwei GA, Moore CE, Willars GB, Tee AR, Herbert TP. Xie J, et al. Cell Signal. 2011 Dec;23(12):1927-35. doi: 10.1016/j.cellsig.2011.06.025. Epub 2011 Jul 6. Cell Signal. 2011. PMID: 21763421 Free PMC article. - Sestrins function as guanine nucleotide dissociation inhibitors for Rag GTPases to control mTORC1 signaling.
Peng M, Yin N, Li MO. Peng M, et al. Cell. 2014 Sep 25;159(1):122-133. doi: 10.1016/j.cell.2014.08.038. Cell. 2014. PMID: 25259925 Free PMC article. - Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex.
Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, Hoxhaj G, Saghatelian A, Shaw RJ, Manning BD. Howell JJ, et al. Cell Metab. 2017 Feb 7;25(2):463-471. doi: 10.1016/j.cmet.2016.12.009. Epub 2017 Jan 12. Cell Metab. 2017. PMID: 28089566 Free PMC article. - LKB1 and AMP-activated protein kinase control of mTOR signalling and growth.
Shaw RJ. Shaw RJ. Acta Physiol (Oxf). 2009 May;196(1):65-80. doi: 10.1111/j.1748-1716.2009.01972.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245654 Free PMC article. Review. - Amino acid regulation of TOR complex 1.
Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Avruch J, et al. Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E592-602. doi: 10.1152/ajpendo.90645.2008. Epub 2008 Sep 2. Am J Physiol Endocrinol Metab. 2009. PMID: 18765678 Free PMC article. Review.
Cited by
- Repurposing metabolic regulators: antidiabetic drugs as anticancer agents.
Dhas Y, Biswas N, M R D, Jones LD, Ashili S. Dhas Y, et al. Mol Biomed. 2024 Sep 28;5(1):40. doi: 10.1186/s43556-024-00204-z. Mol Biomed. 2024. PMID: 39333445 Free PMC article. Review. - Progress in antitumor mechanisms and applications of phenformin (Review).
Zhong Q, Li D, Yang XP. Zhong Q, et al. Oncol Rep. 2024 Nov;52(5):151. doi: 10.3892/or.2024.8810. Epub 2024 Sep 20. Oncol Rep. 2024. PMID: 39301645 Free PMC article. Review. - Role of Autophagy and AMPK in Cancer Stem Cells: Therapeutic Opportunities and Obstacles in Cancer.
Kovale L, Singh MK, Kim J, Ha J. Kovale L, et al. Int J Mol Sci. 2024 Aug 8;25(16):8647. doi: 10.3390/ijms25168647. Int J Mol Sci. 2024. PMID: 39201332 Free PMC article. Review. - Discovering Potential in Non-Cancer Medications: A Promising Breakthrough for Multiple Myeloma Patients.
Al-Odat OS, Nelson E, Budak-Alpdogan T, Jonnalagadda SC, Desai D, Pandey MK. Al-Odat OS, et al. Cancers (Basel). 2024 Jun 28;16(13):2381. doi: 10.3390/cancers16132381. Cancers (Basel). 2024. PMID: 39001443 Free PMC article. Review. - Lomitapide repurposing for treatment of malignancies: A promising direction.
Wu HT, Wu BX, Fang ZX, Wu Z, Hou YY, Deng Y, Cui YK, Liu J. Wu HT, et al. Heliyon. 2024 Jun 13;10(12):e32998. doi: 10.1016/j.heliyon.2024.e32998. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 38988566 Free PMC article. Review.
References
- Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. - PubMed
- Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. - PubMed
- Bertino JR, Carman MD, Weiner HL, Cashmore A, Moroson BA, Srimatkandada S, Schornagel JH, Medina WD, Dube SK. Gene amplification and altered enzymes as mechanisms for the development of drug resistance. Cancer treatment reports. 1983;67:901–904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK078019-02/DK/NIDDK NIH HHS/United States
- U01 CA141464/CA/NCI NIH HHS/United States
- R01 DK073802-04/DK/NIDDK NIH HHS/United States
- CAPMC/ CIHR/Canada
- DK078019/DK/NIDDK NIH HHS/United States
- R01 DK073802/DK/NIDDK NIH HHS/United States
- R01 DK078019/DK/NIDDK NIH HHS/United States
- DK73802/DK/NIDDK NIH HHS/United States
- U01 CA084292/CA/NCI NIH HHS/United States
- U01 CA141464-01/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases