Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival - PubMed (original) (raw)
Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival
Evangelia Emmanouilidou et al. J Neurosci. 2010.
Abstract
alpha-Synuclein is central in Parkinson's disease pathogenesis. Although initially alpha-synuclein was considered a purely intracellular protein, recent data suggest that it can be detected in the plasma and CSF of humans and in the culture media of neuronal cells. To address a role of secreted alpha-synuclein in neuronal homeostasis, we have generated wild-type alpha-synuclein and beta-galactosidase inducible SH-SY5Y cells. Soluble oligomeric and monomeric species of alpha-synuclein are readily detected in the conditioned media (CM) of these cells at concentrations similar to those observed in human CSF. We have found that, in this model, alpha-synuclein is secreted by externalized vesicles in a calcium-dependent manner. Electron microscopy and liquid chromatography-mass spectrometry proteomic analysis demonstrate that these vesicles have the characteristic hallmarks of exosomes, secreted intraluminar vesicles of multivesicular bodies. Application of CM containing secreted alpha-synuclein causes cell death of recipient neuronal cells, which can be reversed after alpha-synuclein immunodepletion from the CM. High- and low-molecular-weight alpha-synuclein species, isolated from this CM, significantly decrease cell viability. Importantly, treatment of the CM with oligomer-interfering compounds before application rescues the recipient neuronal cells from the observed toxicity. Our results show for the first time that cell-produced alpha-synuclein is secreted via an exosomal, calcium-dependent mechanism and suggest that alpha-synuclein secretion serves to amplify and propagate Parkinson's disease-related pathology.
Figures
Figure 1.
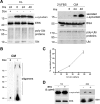
α-Synuclein is constitutively released by a nonclassical secretory pathway. A, SH-SY5Y cells were cultured in the presence or absence of Dox for 8 d in 10% FBS. After that period, the culture medium was replaced with 2% FBS medium for the indicated times. Cell lysates (CL; left) and concentrated CM (right) were analyzed by Western immunoblotting with antibodies to α-synuclein or ubiquitin (Ubi). ERK and BSA levels were used as loading controls. B, SH-SY5Y cells were cultured as in A, and the CM was collected after 48 h and analyzed by Western immunoblotting with C-20 antibody. C, Representative plot showing the levels of secreted α-synuclein, estimated as the percentage ratio of the extracellular α-synuclein versus the intracellular α-synuclein, over time. Each point represents the mean ± SD of three measurements. D, α-Synuclein-expressing cells were treated with 2 μg/ml BFA (+) or without (−) for 6 h. The levels of intracellular α-synuclein (CL) and extracellular α-synuclein (CM) were assessed by Western immunoblotting. ERK and BSA were used as loading controls.
Figure 2.
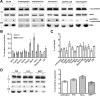
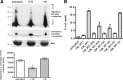
α-Synuclein is released by a calcium-dependent endocytic mechanism. A, Representative immunoblots showing the levels of intracellular and extracellular α-synuclein after no treatment (−) or a 6 h treatment (+) with compounds that either affect Ca2+ concentration or impair the endosomal pathway. Actin and BSA levels were used as loading controls. B, Graph summarizing the effect of various chemical compounds on α-synuclein secretion after the 6 h treatments. Data are shown as mean ± SD (n = 3). *p < 0.05, statistically significant differences (independent t test). C, α-Synuclein-expressing cells were treated with various chemical compounds for 6 h. Cell viability was assessed with EthD-1/Hoechst staining. Data are presented as mean ± SD (n = 3) and statistically analyzed by an independent t test. D, α-Synuclein-expressing cells were treated for 6 h with vehicle, PSI (10 n
m
), H2O2 (0.5 μ
m
), or MPP+ (0.25 m
m
). After treatment, cell homogenates and concentrated CM were analyzed by Western immunoblotting using C-20 antibody. Actin and BSA were used as loading controls. One representative immunoblot from each treatment is shown. The graph is summarizing the results of three independent experiments (n = 3; mean ± SD, one-way ANOVA test followed by Tukey's test). Stauro, Staurosporin; Thapsi, thapsigargin; Meth, methionine; Iono, ionomycin.
Figure 3.
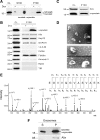
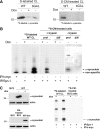
α-Synuclein exocytosis is mediated by membrane vesicles that resemble exosomes. A, α-Synuclein-expressing cells and nonexpressing CTL cells were cultured in 2% FBS for 48 h. After ultracentrifugation of the CM, the membrane (P100) and the soluble (S100) fractions were analyzed for α-synuclein with the polyclonal C-20 antibody. B, P100 pellets were reconstituted in RIPA buffer and analyzed by Western immunoblotting with antibodies against the proteins indicated. TfR, Transferrin receptor. C, P100 pellet representing pure exosomes was analyzed using antibodies against α-synuclein (C-20) and Alix. D, Externalized membrane vesicles were prepared as in A. After fixation, vesicles were negatively stained with 2% uranyl acetate and observed by electron microscopy. Scale bar, 100 nm. E, Analysis of externalized membrane vesicles by mass spectrometry. The annotated product ion MS2 spectrum of the tryptic peptide EGVVQGVASVAEK traceable to α-synuclein is depicted. F, The P100 fraction containing exosomes was treated with Na2CO3. After a 50,000 × g centrifugation, the integral membrane proteins were recovered in the pellet (P), whereas non-integral and lumen proteins remained in the supernatant (S). P and S were analyzed for α-synuclein by immunoblotting with the C-20 antibody.
Figure 4.
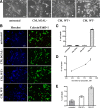
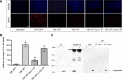
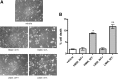
Secreted α-synuclein induces cell death to differentiated SH-SY5Y cells. A, α-Synuclein-expressing and uninduced CTL cells, as well as bGAL-expressing cells, were cultured in 2% FBS for 48 h. CM was collected and applied to differentiated SH-SY5Y cells for 24 h. Representative phase micrographs of the recipient cells are shown. Scale bar, 50 μm. B, CM was prepared and used to treat recipient SH-SY5Y cells as in A. After 24 h, the recipient cells were stained with EthD-1 (red) and Hoechst (blue). Scale bar, 50 μm. C, Percentage cell death was determined by counting the percentage ratio of EthD-1-positive cells versus the Hoechst-positive cells. Quantitative analysis demonstrated a statistically significant increase in cell death when recipient cells were treated with CM from α-synuclein-expressing cells (n = 4; mean ± SD, one-way ANOVA test followed by Tukey's test, *p < 0.001). D, CM was collected from α-synuclein-expressing cells as in A and applied to differentiated SH-SY5Y cells for 24 h. Cell death was quantified by EthD-1/Hoechst staining. The amount of secreted α-synuclein present in the CM was determined by Western immunoblotting and recombinant α-synuclein as standard. The average of three experiments is shown. E, CM from α-synuclein-expressing or uninduced CTL cells were applied to cycling SH-SY5Y cells for 24 h, and the recipient cells were stained with EthD-1 and Hoechst. Quantitative analysis showed a reduction in cell viability from α-synuclein-rich CM (n = 4; mean ± SD, independent t test, *p < 0.01).
Figure 5.
Immunodepletion of α-synuclein from the CM reduces CM-induced toxicity. A, α-Synuclein-expressing and nonexpressing CTL cells were cultured in 2% FBS for 48 h. The CM was cleared from cell debris and immunoprecipitated with antibodies to α-synuclein or c-myc. Immunodepleted and control CM were applied to rat embryonic cortical neurons for 24 h. Recipient neurons were stained with EthD-1 (red) and Hoechst (blue). Scale bar, 10 μm. B, Quantification of the cell death revealed significant increases in cytotoxicity when α-synuclein was present in the CM. Immunodepletion of α-synuclein from the CM was protective against the death phenotype. Data are presented as mean ± SD (n = 3) and analyzed with one-way ANOVA test followed by Tukey's test; *p < 0.001 comparing CM from WT+ with CM from WT−; #p < 0.001 comparing CM from WT− with α-synuclein-immunodepleted CM (WT-/Syn-1 IP). C, Cell lysates (CL) of α-synuclein-expressing (−dox) or nonexpressing CTL (+dox) cells were immunoprecipitated and Western immunoblotted with the Syn-1 antibody. α-Synuclein was successfully immunoprecipitated. α-Synuclein present in the CM of these cells was similarly immunoprecipitated. Under such conditions, the CM was almost completely depleted of α-synuclein (CM/IP/Syn-1). As expected, control immunoprecipitation using the A-14 c-myc antibody (CM/IP/c-myc) had no effect on α-synuclein levels.
Figure 6.
Externalized α-synuclein is uptaken by proliferating SH-SY5Y cells but not neuronal cells. A, α-Synuclein-expressing (−dox) or nonexpressing CTL (+dox) cells and bGAL-expressing (bGAL-dox) cells were 35S labeled. CM from the labeled cells, containing labeled secreted proteins (see Materials and Methods), was applied to recipient cycling SH-SY5Y cells. Endogenously expressed (left) or uptaken (right) labeled α-synuclein was detected in recipient cell extracts by gel autoradiography after immunoprecipitation of the cell lysates with Syn-1 antibody. B, As in A, but c-myc immunoprecipitation was used as a negative control and CM from cells, containing abundant labeled secreted proteins, was applied to both recipient proliferating (prol) and differentiated (diff) SH-SY5Y cells. The recipient cells were either treated with trypsin–EDTA (+trypsin) or washed thoroughly (−trypsin). Gels were exposed for 30 d. C, Left, BV2 cell lysate was analyzed for the presence of α-synuclein by Syn-1 immunoblotting compared with cells expressing (−dox) or nonexpressing (+dox) α-synuclein. CM from α-synuclein-expressing cells (WT-dox) was collected and applied to BV2 cells. Uptake of α-synuclein by recipient BV2 cells was assessed in the cell lysates by Western immunoblotting using the Syn-1 antibody. Actin was used as loading control. Right, α-Synuclein-expressing (−dox) or nonexpressing CTL (+dox) cells were 35S labeled (35S-labeled WT CL). CM from the labeled cells was applied to recipient cycling BV2 cells, which were subsequently treated with trypsin–EDTA (35S-CM-treated BV2 cells). Cell-produced (first and second lanes) or uptaken labeled α-synuclein (third and fourth lanes) was detected in cell extracts by gel autoradiography after immunoprecipitation of the cell lysates with Syn-1 antibody. c-myc immunoprecipitation was used as a negative control (fifth lane).
Figure 7.
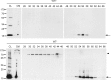
Extracellular α-synuclein oligomeric and monomeric species are present in CM. α-Synuclein-expressing (WT−) and nonexpressing CTL (WT+) cells were cultured in 2% FBS for 48 h. The CM was cleared from cell debris, concentrated, and fractionated by SEC. The cell lysates (CL), the concentrated CM, and the SEC fractions corresponding to WT+ and WT− cells were further analyzed for α-synuclein with the Syn-1 antibody. Arrows show α-synuclein present in LMW fractions, and arrowhead shows α-synuclein present in HMW fractions.
Figure 8.
Treatment of secreted α-synuclein-rich CM with oligomer-interfering compounds reduces CM-induced toxicity. A, α-Synuclein-expressing cells were cultured in 2% FBS for 48 h. The CM was cleared from cell debris and equal parts were treated with vehicle, CR (0.4 μ
m
), or SI (0.4 μ
m
) for 4 h at 4°C. CM was then concentrated and analyzed for α-synuclein with the C-20 antibody. After treatment, the levels of secreted α-synuclein oligomers were quantified (n = 3; mean ± SD, one-way ANOVA test, *p < 0.01). B, CM was collected and treated as in A. After treatment with the compounds, CM was applied to differentiated SH-SY5Y cells for 24 h. Survival was assayed by EthD-1/Hoechst staining. Data are shown as mean ± SD (n = 3). Statistical analysis was performed using the one-way ANOVA test followed by Tukey's test (*p < 0.001 comparing CM, WT+ with CM, WT−; #p < 0.001 comparing CM, WT− with CM, WT−/CR or CM, WT−/SI).
Figure 9.
Isolated secreted α-synuclein oligomeric and monomeric species decrease the viability of differentiated SH-SY5Y cells. A, α-Synuclein-expressing and uninduced CTL cells were cultured in 2% FBS for 48 h. After removal of cell debris, the CM was fractionated by SEC. Extracellular HMW and LMW α-synuclein-containing species were collected, lyophilized, and applied to differentiated SH-SY5Y cells for 48 h. Representative phase micrographs are shown. Scale bar, 50 μm. B, Quantification graph presenting the increase in cell death after application of extracellular HMW or LMW α-synuclein species for 48 h (n = 3; mean ± SD, one-way ANOVA test followed by Tukey's test, *p < 0.001, **p = 0.001).
Similar articles
- Suppression of MAPK attenuates neuronal cell death induced by activated glia-conditioned medium in alpha-synuclein overexpressing SH-SY5Y cells.
Yshii LM, Denadai-Souza A, Vasconcelos AR, Avellar MC, Scavone C. Yshii LM, et al. J Neuroinflammation. 2015 Oct 26;12:193. doi: 10.1186/s12974-015-0412-7. J Neuroinflammation. 2015. PMID: 26502720 Free PMC article. - The protective role of AMP-activated protein kinase in alpha-synuclein neurotoxicity in vitro.
Dulovic M, Jovanovic M, Xilouri M, Stefanis L, Harhaji-Trajkovic L, Kravic-Stevovic T, Paunovic V, Ardah MT, El-Agnaf OM, Kostic V, Markovic I, Trajkovic V. Dulovic M, et al. Neurobiol Dis. 2014 Mar;63:1-11. doi: 10.1016/j.nbd.2013.11.002. Epub 2013 Nov 20. Neurobiol Dis. 2014. PMID: 24269733 - Aggregates assembled from overexpression of wild-type alpha-synuclein are not toxic to human neuronal cells.
Ko LW, Ko HH, Lin WL, Kulathingal JG, Yen SH. Ko LW, et al. J Neuropathol Exp Neurol. 2008 Nov;67(11):1084-96. doi: 10.1097/NEN.0b013e31818c3618. J Neuropathol Exp Neurol. 2008. PMID: 18957893 Free PMC article. - From inflammasome to Parkinson's disease: Does the NLRP3 inflammasome facilitate exosome secretion and exosomal alpha-synuclein transmission in Parkinson's disease?
Si XL, Fang YJ, Li LF, Gu LY, Yin XZ, Jun-Tian, Yan YP, Pu JL, Zhang BR. Si XL, et al. Exp Neurol. 2021 Feb;336:113525. doi: 10.1016/j.expneurol.2020.113525. Epub 2020 Nov 5. Exp Neurol. 2021. PMID: 33161049 Review. - Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Pulliam L, et al. J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
Cited by
- Extracellular Alpha-Synuclein Promotes a Neuroinhibitory Secretory Phenotype in Astrocytes.
Di Marco Vieira B, Radford RAW, Hayashi J, Eaton ED, Greenaway B, Jambas M, Petcu EB, Chung RS, Pountney DL. Di Marco Vieira B, et al. Life (Basel). 2020 Sep 8;10(9):183. doi: 10.3390/life10090183. Life (Basel). 2020. PMID: 32911644 Free PMC article. - Port-to-port delivery: Mobilization of toxic sphingolipids via extracellular vesicles.
Scesa G, Moyano AL, Bongarzone ER, Givogri MI. Scesa G, et al. J Neurosci Res. 2016 Nov;94(11):1333-40. doi: 10.1002/jnr.23798. J Neurosci Res. 2016. PMID: 27638615 Free PMC article. - The Role of Astrocytes in Parkinson's Disease : Astrocytes in Parkinson's Disease.
Garcia R, Zarate S, Srinivasan R. Garcia R, et al. Adv Neurobiol. 2024;39:319-343. doi: 10.1007/978-3-031-64839-7_13. Adv Neurobiol. 2024. PMID: 39190081 Review. - Alpha-Synuclein Oligomers-Neurotoxic Molecules in Parkinson's Disease and Other Lewy Body Disorders.
Ingelsson M. Ingelsson M. Front Neurosci. 2016 Sep 5;10:408. doi: 10.3389/fnins.2016.00408. eCollection 2016. Front Neurosci. 2016. PMID: 27656123 Free PMC article. Review. - Intercellular transmission of alpha-synuclein.
Wu S, Schekman RW. Wu S, et al. Front Mol Neurosci. 2024 Sep 11;17:1470171. doi: 10.3389/fnmol.2024.1470171. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39324117 Free PMC article. Review.
References
- Ahn KJ, Paik SR, Chung KC, Kim J. Amino acid sequence motifs and mechanistic features of the membrane translocation of alpha-synuclein. J Neurochem. 2006;97:265–279. - PubMed
- Albani D, Peverelli E, Rametta R, Batelli S, Veschini L, Negro A, Forloni G. Protective effect of TAT-delivered alpha-synuclein: relevance of the C-terminal domain and involvement of HSP70. FASEB J. 2004;18:1713–1715. - PubMed
- Barclay JW, Morgan A, Burgoyne RD. Calcium-dependent regulation of exocytosis. Cell Calcium. 2005;38:343–353. - PubMed
- Berg D, Holzmann C, Riess O. 14-3-3 proteins in the nervous system. Nat Rev Neurosci. 2003;4:752–762. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources