Structural organization of a filamentous influenza A virus - PubMed (original) (raw)
Structural organization of a filamentous influenza A virus
Lesley J Calder et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Influenza is a lipid-enveloped, pleomorphic virus. We combine electron cryotomography and analysis of images of frozen-hydrated virions to determine the structural organization of filamentous influenza A virus. Influenza A/Udorn/72 virions are capsule-shaped or filamentous particles of highly uniform diameter. We show that the matrix layer adjacent to the membrane is an ordered helix of the M1 protein and its close interaction with the surrounding envelope determines virion morphology. The ribonucleoprotein particles (RNPs) that package the genome segments form a tapered assembly at one end of the virus interior. The neuraminidase, which is present in smaller numbers than the hemagglutinin, clusters in patches and are typically present at the end of the virion opposite to RNP attachment. Incubation of virus at low pH causes a loss of filamentous morphology, during which we observe a structural transition of the matrix layer from its helical, membrane-associated form to a multilayered coil structure inside the virus particle. The polar organization of the virus provides a model for assembly of the virion during budding at the host membrane. Images and tomograms of A/Aichi/68 X-31 virions show the generality of these conclusions to non-filamentous virions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
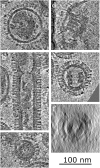
Fig. 1.
Tomogram sections of frozen-hydrated influenza A virions recorded in a single tomogram field. (A_–_G) A/Udorn/72 virions from single field shown in
Fig. S1_A_
and
Movie S1
. Virion in A is identical to that in B, but has been manually colored to indicate features corresponding to RNPs (red), multilayered-coil (blue), NA (purple), and HA (green). (K, L, and M) A/Aichi/68 X-31 virions from single field shown in
Fig. S1b
and
Movie S2
. Negative stain images of (H) HA rosette (green HA trimer model inset), (I) NA rosette (purple NA head domain tetramer model inset), and (J) RNP purified from X-31. Red lines are used for comparison of RNP length. Purple arcs in D and K indicate typical NA clusters at one end.
Fig. 2.
Low-dose images of Udorn virions. (A) Capsule-shaped virion at pH 7 and (B and C) after bromelain digestion.
Fig. 3.
Filamentous influenza A Udorn shows a helical organization of the matrix protein. (A) Image of a virion. (C) Fourier transform of the area within the membrane (black box) in A indicates a helical organization. A lattice (red) shows the prominent reflections arising from one side of the helix. The 38-Å reflection arises from a 7-start helix. (B) Images obtained after two-sided (Upper) and one-sided (Lower) filtering of the transform in C on three layer lines and the equator (indicated by black ticks at right).
Fig. 4.
Tomogram (also shown in
Movies S3
and
S4
) section showing frozen-hydrated influenza A Udorn virions after acid treatment for 10 min at pH 4.9 and trypsin treatment after neutralization.
Fig. 5.
Gallery of multilayered coils from acid and trypsin-treated virions. (A) Spherical virion with multilayered coil axis perpendicular to image and resolved membrane. (B) Filamentous particle showing both intact matrix layer and matrix layer peeling off membrane to form a multilayered coil. (C) Small coil showing layered structure. (D) Multilayered coil viewed perpendicular to axis showing membrane attachment. (E) Side-on view of multilayered coil attached to membrane. (F) Same coil as E viewed down axis.
Similar articles
- Architecture of ribonucleoprotein complexes in influenza A virus particles.
Noda T, Sagara H, Yen A, Takada A, Kida H, Cheng RH, Kawaoka Y. Noda T, et al. Nature. 2006 Jan 26;439(7075):490-2. doi: 10.1038/nature04378. Nature. 2006. PMID: 16437116 - Structure of influenza virus ribonucleoprotein complexes and their packaging into virions.
Noda T, Kawaoka Y. Noda T, et al. Rev Med Virol. 2010 Nov;20(6):380-91. doi: 10.1002/rmv.666. Rev Med Virol. 2010. PMID: 20853340 Free PMC article. Review. - Structural Analysis of the Roles of Influenza A Virus Membrane-Associated Proteins in Assembly and Morphology.
Chlanda P, Schraidt O, Kummer S, Riches J, Oberwinkler H, Prinz S, Kräusslich HG, Briggs JA. Chlanda P, et al. J Virol. 2015 Sep;89(17):8957-66. doi: 10.1128/JVI.00592-15. Epub 2015 Jun 17. J Virol. 2015. PMID: 26085153 Free PMC article. - Structural studies of influenza virus RNPs by electron microscopy indicate molecular contortions within NP supra-structures.
Gallagher JR, Torian U, McCraw DM, Harris AK. Gallagher JR, et al. J Struct Biol. 2017 Mar;197(3):294-307. doi: 10.1016/j.jsb.2016.12.007. Epub 2016 Dec 19. J Struct Biol. 2017. PMID: 28007449 Free PMC article. - Influenza virus assembly and budding.
Rossman JS, Lamb RA. Rossman JS, et al. Virology. 2011 Mar 15;411(2):229-36. doi: 10.1016/j.virol.2010.12.003. Epub 2011 Jan 14. Virology. 2011. PMID: 21237476 Free PMC article. Review.
Cited by
- Electron Tomography as a Tool to Study SARS-CoV-2 Morphology.
Wu H, Fujioka Y, Sakaguchi S, Suzuki Y, Nakano T. Wu H, et al. Int J Mol Sci. 2024 Nov 1;25(21):11762. doi: 10.3390/ijms252111762. Int J Mol Sci. 2024. PMID: 39519314 Free PMC article. Review. - Recombinant Influenza A Viruses Expressing Reporter Genes from the Viral NS Segment.
Martinez-Sobrido L, Nogales A. Martinez-Sobrido L, et al. Int J Mol Sci. 2024 Oct 1;25(19):10584. doi: 10.3390/ijms251910584. Int J Mol Sci. 2024. PMID: 39408912 Free PMC article. Review. - Headless hemagglutinin-containing influenza viral particles direct immune responses toward more conserved epitopes.
Hamele CE, Luo Z, Leonard RA, Spurrier MA, Burke KN, Webb SR, Rountree W, Li Z, Heaton BE, Heaton NS. Hamele CE, et al. J Virol. 2024 Oct 22;98(10):e0116624. doi: 10.1128/jvi.01166-24. Epub 2024 Sep 26. J Virol. 2024. PMID: 39324791 - Assembly of respiratory syncytial virus matrix protein lattice and its coordination with fusion glycoprotein trimers.
Sibert BS, Kim JY, Yang JE, Ke Z, Stobart CC, Moore ML, Wright ER. Sibert BS, et al. Nat Commun. 2024 Jul 14;15(1):5923. doi: 10.1038/s41467-024-50162-x. Nat Commun. 2024. PMID: 39004634 Free PMC article. - The Influenza A Virus Replication Cycle: A Comprehensive Review.
Carter T, Iqbal M. Carter T, et al. Viruses. 2024 Feb 19;16(2):316. doi: 10.3390/v16020316. Viruses. 2024. PMID: 38400091 Free PMC article. Review.
References
- Jackson DC, et al. Electron microscopic evidence for the association of M2 protein with the influenza virion. Arch Virol. 1991;118:199–207. - PubMed
- Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. - PubMed
- Wharton SA, Belshe RB, Skehel JJ, Hay AJ. Role of virion M2 protein in influenza virus uncoating: Specific reduction in the rate of membrane fusion between virus and liposomes by amantadine. J Gen Virol. 1994;75:945–948. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources