Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians - PubMed (original) (raw)
. 2010 May 17;5(5):e10667.
doi: 10.1371/journal.pone.0010667.
Lotta Nylund, Marco Candela, Rita Ostan, Laura Bucci, Elisa Pini, Janne Nikkïla, Daniela Monti, Reetta Satokari, Claudio Franceschi, Patrizia Brigidi, Willem De Vos
Affiliations
- PMID: 20498852
- PMCID: PMC2871786
- DOI: 10.1371/journal.pone.0010667
Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians
Elena Biagi et al. PLoS One. 2010.
Erratum in
- PLoS One. 2010;5(6). doi: 10.1371/annotation/df45912f-d15c-44ab-8312-e7ec0607604d
Abstract
Background: Age-related physiological changes in the gastrointestinal tract, as well as modifications in lifestyle, nutritional behaviour, and functionality of the host immune system, inevitably affect the gut microbiota, resulting in a greater susceptibility to infections.
Methodology/principal findings: By using the Human Intestinal Tract Chip (HITChip) and quantitative PCR of 16S rRNA genes of Bacteria and Archaea, we explored the age-related differences in the gut microbiota composition among young adults, elderly, and centenarians, i.e subjects who reached the extreme limits of the human lifespan, living for over 100 years. We observed that the microbial composition and diversity of the gut ecosystem of young adults and seventy-years old people is highly similar but differs significantly from that of the centenarians. After 100 years of symbiotic association with the human host, the microbiota is characterized by a rearrangement in the Firmicutes population and an enrichment in facultative anaerobes, notably pathobionts. The presence of such a compromised microbiota in the centenarians is associated with an increased inflammatory status, also known as inflammageing, as determined by a range of peripheral blood inflammatory markers. This may be explained by a remodelling of the centenarians' microbiota, with a marked decrease in Faecalibacterium prauznitzii and relatives, symbiotic species with reported anti-inflammatory properties. As signature bacteria of the long life we identified specifically Eubacterium limosum and relatives that were more than ten-fold increased in the centenarians.
Conclusions/significance: We provide evidence for the fact that the ageing process deeply affects the structure of the human gut microbiota, as well as its homeostasis with the host's immune system. Because of its crucial role in the host physiology and health status, age-related differences in the gut microbiota composition may be related to the progression of diseases and frailty in the elderly population.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
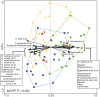
Figure 1. Hierarchical clustering, with heat map, of the HITChip profiles of centenarians, elderly and young adults.
Subjects belonging to the groups C, E, and Y are indicated by green, blue, and yellow squares, respectively. Darkness of the spot corresponds to the bacterial abundance in the sample. Pearson correlation and Ward's clustering method were used. Genus-like bacterial groups belonging to Proteobacteria (P), Bacilli (B), Clostridium cluster IV (C.IV) and XIVa (C.XIVa) are indicated. The two phylogenetic groups members of the Clostridium cluster IV located within the Clostridium cluster XIVa (bottom) are Faecalibacterium prausnitzii et rel. and Papillibacter cinnamovorans et rel.
Figure 2. Triplot of the RDA of the microbiota composition of centenarians, elderly and young adults.
Subjects belonging to group C, E, and Y are indicated by green circles, blue squares and yellow diamonds, respectively. Constrained explanatory variables (C, E, and Y) are indicated by red triangles. Responding bacterial subgroups that explained more than 10% of the variability of the samples are indicated by black arrows. First and second ordination axes are plotted, showing 5.2% and 0.9% of the variability in the dataset, respectively. Log transformed data were used for the analysis. Bottom-left, P value obtained by MCPP is reported. Abbreviations: C., Clostridium; E., Eubacterium; F., Faecalibacterium; R., Ruminococcus; K., Klebsiella.
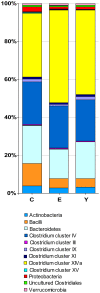
Figure 3. Relative contribution of the phylum/order-like phylogroups to the microbiota of centenarians, elderly and young adults.
In the legend, phylum/order-like phylogroups which contribute for at least 0.5% to one of the profiles are indicated.
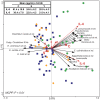
Figure 4. Correlation between microbiota composition and plasma levels of pro-inflammatory cytokines.
In the RDA blood cytokine levels (red arrows) and age groups (C, S, and Y, red triangles) are used as linear and nominal environmental variables, respectively. Samples belonging to C, S and Y groups are indicated by green circles, blue squares and yellow diamonds, respectively. Responding bacterial subgroups that explained more than 20% of the variability of the samples are indicated by black arrows. First and second ordination axes are plotted, showing 5.8% and 3.1% of the variability in the dataset, respectively. Red arrows which are not labelled corresponds to (clockwise, starting from the left) TNF-α, IFN-γ, IL-2, IL-1α, IL-12p70, and IL-1β. Log transformed data were used for this analysis. Bottom-left, P value obtained by MCPP is reported. Top-left, average blood levels of IL-6 and IL-8 in groups C, S and Y are reported.
Similar articles
- Cultivable and pyrosequenced fecal microflora in centenarians and young subjects.
Drago L, Toscano M, Rodighiero V, De Vecchi E, Mogna G. Drago L, et al. J Clin Gastroenterol. 2012 Oct;46 Suppl:S81-4. doi: 10.1097/MCG.0b013e3182693982. J Clin Gastroenterol. 2012. PMID: 22955365 - Aging of the human metaorganism: the microbial counterpart.
Biagi E, Candela M, Fairweather-Tait S, Franceschi C, Brigidi P. Biagi E, et al. Age (Dordr). 2012 Feb;34(1):247-67. doi: 10.1007/s11357-011-9217-5. Epub 2011 Feb 24. Age (Dordr). 2012. PMID: 21347607 Free PMC article. Review. - Comparison of the Gut Microbiota of Centenarians in Longevity Villages of South Korea with Those of Other Age Groups.
Kim BS, Choi CW, Shin H, Jin SP, Bae JS, Han M, Seo EY, Chun J, Chung JH. Kim BS, et al. J Microbiol Biotechnol. 2019 Mar 28;29(3):429-440. doi: 10.4014/jmb.1811.11023. J Microbiol Biotechnol. 2019. PMID: 30661321 - Age-Related Variation of Bacterial and Fungal Communities in Different Body Habitats across the Young, Elderly, and Centenarians in Sardinia.
Wu L, Zeng T, Deligios M, Milanesi L, Langille MGI, Zinellu A, Rubino S, Carru C, Kelvin DJ. Wu L, et al. mSphere. 2020 Feb 26;5(1):e00558-19. doi: 10.1128/mSphere.00558-19. mSphere. 2020. PMID: 32102941 Free PMC article. - Understanding gut microbiota in elderly's health will enable intervention through probiotics.
Pérez Martínez G, Bäuerl C, Collado MC. Pérez Martínez G, et al. Benef Microbes. 2014 Sep;5(3):235-46. doi: 10.3920/BM2013.0079. Benef Microbes. 2014. PMID: 24889891 Review.
Cited by
- Colonic Transit Time Is a Driven Force of the Gut Microbiota Composition and Metabolism: In Vitro Evidence.
Tottey W, Feria-Gervasio D, Gaci N, Laillet B, Pujos E, Martin JF, Sebedio JL, Sion B, Jarrige JF, Alric M, Brugère JF. Tottey W, et al. J Neurogastroenterol Motil. 2017 Jan 30;23(1):124-134. doi: 10.5056/jnm16042. J Neurogastroenterol Motil. 2017. PMID: 27530163 Free PMC article. - Host Transcriptome and Microbiota Signatures Prior to Immunization Profile Vaccine Humoral Responsiveness.
Gonçalves E, Guillén Y, Lama JR, Sanchez J, Brander C, Paredes R, Combadière B. Gonçalves E, et al. Front Immunol. 2021 May 10;12:657162. doi: 10.3389/fimmu.2021.657162. eCollection 2021. Front Immunol. 2021. PMID: 34040607 Free PMC article. Clinical Trial. - Dysbiosis of gut microbiota in inflammatory bowel disease: Current therapies and potential for microbiota-modulating therapeutic approaches.
Alshehri D, Saadah O, Mosli M, Edris S, Alhindi R, Bahieldin A. Alshehri D, et al. Bosn J Basic Med Sci. 2021 Jun 1;21(3):270-283. doi: 10.17305/bjbms.2020.5016. Bosn J Basic Med Sci. 2021. PMID: 33052081 Free PMC article. Review. - Gut Microbiome Composition is Associated with Age and Memory Performance in Pet Dogs.
Kubinyi E, Bel Rhali S, Sándor S, Szabó A, Felföldi T. Kubinyi E, et al. Animals (Basel). 2020 Aug 24;10(9):1488. doi: 10.3390/ani10091488. Animals (Basel). 2020. PMID: 32846928 Free PMC article. - Controlling Gut Microbiota by Twendee X® May Contribute to Dementia Prevention.
You F, Harakawa Y, Yoshikawa T, Inufusa H. You F, et al. Int J Mol Sci. 2023 Nov 23;24(23):16642. doi: 10.3390/ijms242316642. Int J Mol Sci. 2023. PMID: 38068966 Free PMC article.
References
- Imahori K. How I understand ageing. Nutr Rev. 1992;50:351–352. - PubMed
- Kleessen B, Sycura B, Zunft HJ, Blaut M. Affects of inulin and lactose on fecal microflora, microbial activity and bowel habits in elderly constipated persons. Am J Clin Nutr. 1997;65:1397–1402. - PubMed
- Macfarlane GT, Cummings JH, Macfarlane S, Gibson GR. Influence of retention time on degradation of pancreatic enzymes by human colonic bacteria grown in a 3-stage continuous culture system. J Appl Bacteriol. 1989;67:520–527. - PubMed
- Ostan R, Bucci L, Capri M, Salvioli S, Scurti M, et al. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation. 2008;15:224–240. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials