Guidelines for the welfare and use of animals in cancer research - PubMed (original) (raw)
Practice Guideline
. 2010 May 25;102(11):1555-77.
doi: 10.1038/sj.bjc.6605642.
E O Aboagye, F Balkwill, A Balmain, G Bruder, D J Chaplin, J A Double, J Everitt, D A H Farningham, M J Glennie, L R Kelland, V Robinson, I J Stratford, G M Tozer, S Watson, S R Wedge, S A Eccles; Committee of the National Cancer Research Institute
Affiliations
- PMID: 20502460
- PMCID: PMC2883160
- DOI: 10.1038/sj.bjc.6605642
Practice Guideline
Guidelines for the welfare and use of animals in cancer research
P Workman et al. Br J Cancer. 2010.
Abstract
Animal experiments remain essential to understand the fundamental mechanisms underpinning malignancy and to discover improved methods to prevent, diagnose and treat cancer. Excellent standards of animal care are fully consistent with the conduct of high quality cancer research. Here we provide updated guidelines on the welfare and use of animals in cancer research. All experiments should incorporate the 3Rs: replacement, reduction and refinement. Focusing on animal welfare, we present recommendations on all aspects of cancer research, including: study design, statistics and pilot studies; choice of tumour models (e.g., genetically engineered, orthotopic and metastatic); therapy (including drugs and radiation); imaging (covering techniques, anaesthesia and restraint); humane endpoints (including tumour burden and site); and publication of best practice.
Figures
Figure 1
An illustrative process for tumour model selection and use. This representative schema provides an illustration of factors to be considered when designing an animal study. In this particular example, all the factors listed at a given stage (and potentially others) should be considered before moving down, stepwise, to the next stage. Here, an initial consideration is that the choice of model may be based on the relevant molecular status, clinical tumour type or in vitro studies. At the next stage, the animal host will be dictated by the need for, say, a human tumour xenograft versus a genetically engineered mouse model, which have advantages discussed in the text. Considerations of tumour environment and site then follow, after which, in therapy studies, are dosing and endpoint aspects. Note that this schema is illustrative and not prescriptive and that each study must be tailored to the specific scientific question and experimental objectives, with appropriate humane endpoints always applied and pilot studies carried out as needed.
Figure 2
Example of a drug discovery test cascade for identifying small-molecule antitumour drugs. A representative test cascade for identifying a potential small-molecule drug against a given target is shown. A subset of a compound library is initially screened vs the target in vitro, in recombinant protein or cellular assays, using high-throughput automation to identify ‘hits’. Subsequent leads are examined in more detail by assessing their effect on downstream molecular events in cells and their selectivity vs other proteins. A battery of additional in vitro tests is also used for measurement or prediction of physical properties and pharmacokinetic parameters. Only compounds with a promising balance of features are progressed to in vivo testing, usually in mice. Pharmacokinetic (PK) studies, used to understand drug exposure, may initially involve co-inoculation of low doses of compounds (‘cassette dosing’) to minimise animal usage. The tolerability of leads with favourable PK is then assessed at higher doses, before evaluating their pharmacodynamic (PD) effect on tumour and normal tissues at well-tolerated doses. Compounds that do not meet the anticipated level of performance at any stage may result in subsequent rounds of iterative medicinal chemistry to generate improved leads. Selected leads are progressed to efficacy testing to determine the link between target inhibition and the effect on tumour growth or spread (metastasis). Safety studies on late-stage leads are also required before a candidate drug can be selected for examination in cancer patients (not covered here). The application of the test cascade means that compounds are filtered by the earlier stage assays so that a smaller number of compounds, and only those of higher quality, are taken into later stage in vivo assays in animals.
Figure 3
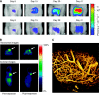
Examples of technologies used in animals for therapeutic cancer research. In vivo tumour models have an essential role in the development of new cancer medicines, enabling the temporal and quantitative effects of treatment to be examined on tumour and normal tissues in the intact organism. Methods used include those to examine (clockwise from far left) molecular determinants of sensitivity to treatment (initially in vitro, corroborated in vivo) such as (a) gene mutations by sequencing, or (b) gene amplification by fluorescent in situ hybridisation; detection of target phospho-epitopes and their inhibition in tumour tissue as determined by: (c) immunohistochemistry or (d) western blotting of cell lysates; (e) tumour vascular density and maturation by fluorescent immunohistochemistry; (f) tumour mRNA expression by gene array analysis with hierarchical clustering of information; (g) imaging techniques such as dynamic contrast-enhanced MRI to measure tumour haemodynamics; and (h) pharmacokinetic analysis of drug concentrations in plasma by mass spectrometry.
Figure 4
Examples of in vivo imaging in pre-clinical cancer research. (A) Optical surface bioluminescence imaging of orthotopically xenografted human PC3 prostate carcinoma cells transfected with luciferase (PC3luc2a). Mice were imaged using a Charged Coupled Device (CCD) camera, which is super-cooled to enhance detection sensitivity and image resolution. The images shown were taken after systemic administration of luciferin, with ‘intensity of luminescence’ shown as ‘heat’ maps and red as maximum intensity. The scale shows the number of photons detected. Top panel: Untreated mice at day 8–41 after transplantation; bottom panel: before and after treatment with 5 mg kg−1 taxotere on day 10. This technique is useful for monitoring treatment effects in deep-seated tumour sites. Light scattering through tissues makes precise quantitation difficult. (B) PET imaging of tumour cell proliferation using 18F-3′-fluoro-3′-deoxy-
L
-thymidine (FLT). Transverse and coronal (0.5 mm) images of HCT116 tumour-bearing mice 24 h before treatment and after 4 daily treatments with the histone deacetylase inhibitor LAQ842 at 25 mg kg−1. 30- to 60-min summed images from a dynamic scan are presented. Numerous radiotracers are available for investigating specific biochemical pathways in vivo, if specialised facilities are available. The scale shows the intensity of radiotracer uptake. (C) Intravital imaging of tumour vasculature of the P22 rat sarcoma growing in a dorsal skin flap window chamber. The image was obtained by multi-photon fluorescence microscopy after i.v. administration of 70 kDa FITC–dextran. High spatial resolution is obtained but surgical intervention is required.
Comment in
- Of mice and men: the evolution of animal welfare guidelines for cancer research.
Dey N, De P, Smith BR, Leyland-Jones B. Dey N, et al. Br J Cancer. 2010 May 25;102(11):1553-4. doi: 10.1038/sj.bjc.6605692. Br J Cancer. 2010. PMID: 20502459 Free PMC article. No abstract available.
Similar articles
- Of mice and men: the evolution of animal welfare guidelines for cancer research.
Dey N, De P, Smith BR, Leyland-Jones B. Dey N, et al. Br J Cancer. 2010 May 25;102(11):1553-4. doi: 10.1038/sj.bjc.6605692. Br J Cancer. 2010. PMID: 20502459 Free PMC article. No abstract available. - Opportunities for improving animal welfare in rodent models of epilepsy and seizures.
Lidster K, Jefferys JG, Blümcke I, Crunelli V, Flecknell P, Frenguelli BG, Gray WP, Kaminski R, Pitkänen A, Ragan I, Shah M, Simonato M, Trevelyan A, Volk H, Walker M, Yates N, Prescott MJ. Lidster K, et al. J Neurosci Methods. 2016 Feb 15;260:2-25. doi: 10.1016/j.jneumeth.2015.09.007. Epub 2015 Sep 12. J Neurosci Methods. 2016. PMID: 26376175 Review. - Lab-animal battle reaches truce.
Abbott A. Abbott A. Nature. 2010 Apr 15;464(7291):964. doi: 10.1038/464964a. Nature. 2010. PMID: 20393527 No abstract available. - Introduction: mitigating risk, facilitating research.
Bayne KA, Garnett NL. Bayne KA, et al. ILAR J. 2008;49(4):369-71. doi: 10.1093/ilar.49.4.369. ILAR J. 2008. PMID: 18849589 No abstract available. - Animal welfare in studies on murine tuberculosis: assessing progress over a 12-year period and the need for further improvement.
Franco NH, Correia-Neves M, Olsson IA. Franco NH, et al. PLoS One. 2012;7(10):e47723. doi: 10.1371/journal.pone.0047723. Epub 2012 Oct 26. PLoS One. 2012. PMID: 23110093 Free PMC article. Review.
Cited by
- Bicalutamide-induced hypoxia potentiates RUNX2-mediated Bcl-2 expression resulting in apoptosis resistance.
Browne G, Nesbitt H, Ming L, Stein GS, Lian JB, McKeown SR, Worthington J. Browne G, et al. Br J Cancer. 2012 Nov 6;107(10):1714-21. doi: 10.1038/bjc.2012.455. Epub 2012 Oct 16. Br J Cancer. 2012. PMID: 23073173 Free PMC article. - NGR-TNF, a novel vascular-targeting agent, does not induce cytokine recruitment of proangiogenic bone marrow-derived cells.
Di Matteo P, Hackl C, Jedeszko C, Valentinis B, Bordignon C, Traversari C, Kerbel RS, Rizzardi GP. Di Matteo P, et al. Br J Cancer. 2013 Jul 23;109(2):360-9. doi: 10.1038/bjc.2013.347. Epub 2013 Jul 4. Br J Cancer. 2013. PMID: 23828516 Free PMC article. - Anti-tumour efficacy of capecitabine in a genetically engineered mouse model of pancreatic cancer.
Courtin A, Richards FM, Bapiro TE, Bramhall JL, Neesse A, Cook N, Krippendorff BF, Tuveson DA, Jodrell DI. Courtin A, et al. PLoS One. 2013 Jun 28;8(6):e67330. doi: 10.1371/journal.pone.0067330. Print 2013. PLoS One. 2013. PMID: 23840665 Free PMC article. - Drug discovery in prostate cancer mouse models.
Valkenburg KC, Pienta KJ. Valkenburg KC, et al. Expert Opin Drug Discov. 2015;10(9):1011-24. doi: 10.1517/17460441.2015.1052790. Epub 2015 Jun 1. Expert Opin Drug Discov. 2015. PMID: 26027638 Free PMC article. Review. - Paclitaxel resistance increases oncolytic adenovirus efficacy via upregulated CAR expression and dysfunctional cell cycle control.
Ingemarsdotter CK, Tookman LA, Browne A, Pirlo K, Cutts R, Chelela C, Khurrum KF, Leung EY, Dowson S, Webber L, Khan I, Ennis D, Syed N, Crook TR, Brenton JD, Lockley M, McNeish IA. Ingemarsdotter CK, et al. Mol Oncol. 2015 Apr;9(4):791-805. doi: 10.1016/j.molonc.2014.12.007. Epub 2014 Dec 29. Mol Oncol. 2015. PMID: 25560085 Free PMC article.
References
- Ahsan H, Aziz MH, Ahmad N (2005) Ultraviolet B exposure activates Stat3 signaling via phosphorylation at tyrosine705 in skin of SKH1 hairless mouse: a target for the management of skin cancer? Biochem Biophys Res Commun 333: 241–246 - PubMed
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA (2000) Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406: 641–645 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 10337/CRUK_/Cancer Research UK/United Kingdom
- 9993/CRUK_/Cancer Research UK/United Kingdom
- G1000121/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- G0500366/MRC_/Medical Research Council/United Kingdom
- 10345/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources