Differential effects of the putative GBF1 inhibitors Golgicide A and AG1478 on enterovirus replication - PubMed (original) (raw)
Differential effects of the putative GBF1 inhibitors Golgicide A and AG1478 on enterovirus replication
Lonneke van der Linden et al. J Virol. 2010 Aug.
Abstract
The genus Enterovirus, belonging to the family Picornaviridae, includes well-known pathogens, such as poliovirus, coxsackievirus, and rhinovirus. Brefeldin A (BFA) impedes replication of several enteroviruses through inhibition of Golgi-specific BFA resistance factor 1 (GBF1), a regulator of secretory pathway integrity and transport. GBF1 mediates the GTP exchange of Arf1, which in activated form recruits coatomer protein complex I (COP-I) to Golgi vesicles, a process important in transport between the endoplasmic reticulum and Golgi vesicles. Recently, the drugs AG1478 and Golgicide A (GCA) were put forward as new inhibitors of GBF1. In this study, we investigated the effects of these putative GBF1 inhibitors on secretory pathway function and enterovirus replication. We show that both drugs induced fragmentation of the Golgi vesicles and caused dissociation of Arf1 and COP-I from Golgi membranes, yet they differed in their effect on GBF1 localization. The effects of AG1478, but not those of GCA, could be countered by overexpression of Arf1, indicating a difference in their molecular mechanism of action. Consistent with this idea, we observed that GCA drastically reduced replication of coxsackievirus B3 (CVB3) and other human enterovirus species, whereas AG1478 had no effect at all on enterovirus replication. Time-of-addition studies and analysis of RNA replication using a subgenomic replicon both showed that GCA suppresses RNA replication of CVB3, which could be countered by overexpression of GBF1. These results indicate that, in contrast to AG1478, GCA inhibits CVB3 RNA replication by targeting GBF1. AG1478 and GCA may be valuable tools to further dissect enterovirus replication.
Figures
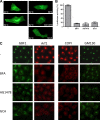
FIG. 1.
AG1478 and GCA disturb the secretory pathway. (A) BGM cells were transfected with pVSVG3(ts045)-GFP, a construct encoding a temperature-sensitive GFP-tagged mutant of the VSV-G protein, and incubated at 40°C. At this temperature, the VSV-G protein is folded incorrectly and retained in the ER. At 30 min after the addition of BFA, AG1478, or GCA, the temperature was switched to the permissive temperature of 32°C for 2 h, which allows the VSV-G protein to fold properly and traffic through the secretory pathway to the plasma membrane. (B) Cells expressing Gaussia luciferase (Gaussia-luc) were treated with BFA, AG1478, or GCA for 2.5 h. Subsequently, the medium was analyzed for luciferase activity. The experiment was performed in triplicate, and the bars represent means ± standard error of the means (SEMs) (error bars). Upon drug treatment, a significant reduction in luciferase activity was detected in comparison with untreated cells (−) (P < 0.001, Student's t test). (C) BGM cells were treated with AG1478, GCA, or BFA for 1 h, after which cells were fixed and stained with antibodies against GBF1, Arf1, COP-I, or GM130.
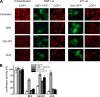
FIG. 2.
Overexpression of GBF1 counters the effects of BFA, GCA, and AG1478, while overexpression of Arf1 counters only the effects of AG1478. (A) Plasmids encoding GBF1 or Arf1 were transfected into BGM cells. At 24 h posttransfection, the cells were treated for 1 h with BFA, AG1478, or GCA. Subsequently, the cells were fixed and stained with an antibody against COP-I. GBF1-wt, wild-type GBF1. (B) A Gaussia-luc construct was cotransfected with GBF1-M832L or Arf1 constructs into cells. The next day, the medium was replaced with (drug-containing) medium, and after 2.5 h, the supernatant was analyzed for luciferase activity. Gaussia-luc activity is expressed as a percentage of luciferase activity in the supernatant of untreated cells. The experiment was performed in triplicate, and the bars represent means ± SEMs (error bars).
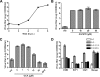
FIG. 3.
GCA, not AG1478, inhibits replication of enteroviruses. (A) BGM cells were infected with CVB3 at an MOI of 5. Total virus titers were determined at the indicated time points. The virus titer was measured as log 50% cell culture infective doses per ml (log CCID50/ml). (B) Various concentrations of AG1478 were added to the cells immediately after infection with CVB3. Virus titers were determined after 8 h. (C) The cells were infected with CVB3 and treated with various concentrations of GCA. Concentrations of 10 μM GCA or higher significantly inhibited CVB3 replication (P < 0.001, one-way analysis of variance [ANOVA]). (D) BGM, HeLa, or BHK-21 cells were infected with CVB3, EV71, CVA21, or mengovirus and treated with GCA or BFA. Virus titers were determined after 8 h. Experiments were performed in triplicate, and the bars represent means ± SEMs (error bars).
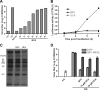
FIG. 4.
GCA inhibits enterovirus RNA replication by targeting GBF1. (A) BGM cells were infected with CVB3 at an MOI of 5. GCA was added to the cells at 1-h intervals, and virus yields were determined after 8 h. (B) Cells were transfected with RNA in vitro transcribed from the p53CB3-LUC subgenomic replicon and subsequently treated with GCA or BFA. At 2, 4, 6, or 8 h.p.i., the cells were lysed, and firefly luciferase activity (in relative light units [RLU]) was determined as a measure of RNA replication. (C) BGM cells were infected with CVB3 at an MOI of 50. At 5.5 h.p.i., the cells were starved in methionine-free medium, and then [35S]methionine and drugs were added to the cells. After 30 min, cells were lysed and proteins were analyzed by SDS-PAGE. (D) Cells transfected with plasmids encoding GBF1-M832L or EGFP as a negative control were infected with CVB3 in the presence of 2 μg/ml BFA, 10 μM GCA, or 30 μM GCA or in the absence of drugs. Virus yields were determined at 8 h.p.i. The experiment was performed in triplicate, and bars represent means ± SEMs.
Similar articles
- GBF1, a guanine nucleotide exchange factor for Arf, is crucial for coxsackievirus B3 RNA replication.
Lanke KH, van der Schaar HM, Belov GA, Feng Q, Duijsings D, Jackson CL, Ehrenfeld E, van Kuppeveld FJ. Lanke KH, et al. J Virol. 2009 Nov;83(22):11940-9. doi: 10.1128/JVI.01244-09. Epub 2009 Sep 9. J Virol. 2009. PMID: 19740986 Free PMC article. - Recruitment of PI4KIIIβ to coxsackievirus B3 replication organelles is independent of ACBD3, GBF1, and Arf1.
Dorobantu CM, van der Schaar HM, Ford LA, Strating JR, Ulferts R, Fang Y, Belov G, van Kuppeveld FJ. Dorobantu CM, et al. J Virol. 2014 Mar;88(5):2725-36. doi: 10.1128/JVI.03650-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352456 Free PMC article. - Rab1b-GBF1-ARF1 Secretory Pathway Axis Is Required for Birnavirus Replication.
Gimenez MC, Frontini-Lopez YR, Pocognoni CA, Roldán JS, García Samartino C, Uhart M, Colombo MI, Terebiznik MR, Delgui LR. Gimenez MC, et al. J Virol. 2022 Feb 23;96(4):e0200521. doi: 10.1128/JVI.02005-21. Epub 2021 Dec 8. J Virol. 2022. PMID: 34878889 Free PMC article. - ARF1 with Sec7 Domain-Dependent GBF1 Activates Coatomer Protein I To Support Classical Swine Fever Virus Entry.
Zhang L, Wang T, Yi Y, Song M, Jin M, Guo K, Zhang Y. Zhang L, et al. J Virol. 2022 Mar 23;96(6):e0219321. doi: 10.1128/jvi.02193-21. Epub 2022 Jan 19. J Virol. 2022. PMID: 35044210 Free PMC article. - Role of the Guanine Nucleotide Exchange Factor GBF1 in the Replication of RNA Viruses.
Martínez JL, Arias CF. Martínez JL, et al. Viruses. 2020 Jun 24;12(6):682. doi: 10.3390/v12060682. Viruses. 2020. PMID: 32599855 Free PMC article. Review.
Cited by
- The development of resistance to an inhibitor of a cellular protein reveals a critical interaction between the enterovirus protein 2C and a small GTPase Arf1.
Viktorova EG, Gabaglio S, Moghimi S, Zimina A, Wynn BG, Sztul E, Belov GA. Viktorova EG, et al. PLoS Pathog. 2023 Sep 18;19(9):e1011673. doi: 10.1371/journal.ppat.1011673. eCollection 2023 Sep. PLoS Pathog. 2023. PMID: 37721955 Free PMC article. - Direct-Acting Antivirals and Host-Targeting Approaches against Enterovirus B Infections: Recent Advances.
Tammaro C, Guida M, Appetecchia F, Biava M, Consalvi S, Poce G. Tammaro C, et al. Pharmaceuticals (Basel). 2023 Jan 29;16(2):203. doi: 10.3390/ph16020203. Pharmaceuticals (Basel). 2023. PMID: 37259352 Free PMC article. Review. - AG1478 Elicits a Novel Anti-Influenza Function via an EGFR-Independent, GBF1-Dependent Pathway.
Zhou X, Zhu L, Bondy C, Wang J, Luo Q, Chen Y. Zhou X, et al. Int J Mol Sci. 2022 May 16;23(10):5557. doi: 10.3390/ijms23105557. Int J Mol Sci. 2022. PMID: 35628375 Free PMC article. - Fighting Enteroviral Infections to Prevent Type 1 Diabetes.
Nekoua MP, Mercier A, Alhazmi A, Sane F, Alidjinou EK, Hober D. Nekoua MP, et al. Microorganisms. 2022 Apr 1;10(4):768. doi: 10.3390/microorganisms10040768. Microorganisms. 2022. PMID: 35456818 Free PMC article. - Picornaviruses: A View from 3A.
Jackson T, Belsham GJ. Jackson T, et al. Viruses. 2021 Mar 11;13(3):456. doi: 10.3390/v13030456. Viruses. 2021. PMID: 33799649 Free PMC article. Review.
References
- Claude, A., B. P. Zhao, C. E. Kuziemsky, S. Dahan, S. J. Berger, J. P. Yan, A. D. Armold, E. M. Sullivan, and P. Melancon. 1999. GBF1: a novel Golgi-associated BFA-resistant guanine nucleotide exchange factor that displays specificity for ADP-ribosylation factor 5. J. Cell Biol. 146:71-84. - PMC - PubMed
- De Palma, A. M., H. J. Thibaut, L. van der Linden, K. Lanke, W. Heggermont, S. Ireland, R. Andrews, M. Arimilli, T. H. Al-Tel, E. De Clercq, F. van Kuppeveld, and J. Neyts. 2009. Mutations in the nonstructural protein 3A confer resistance to the novel enterovirus replication inhibitor TTP-8307. Antimicrob. Agents Chemother. 53:1850-1857. - PMC - PubMed
- Duijsings, D., K. H. Lanke, S. H. van Dooren, M. M. van Dommelen, R. Wetzels, F. de Mattia, E. Wessels, and F. J. van Kuppeveld. 2009. Differential membrane association properties and regulation of class I and class II Arfs. Traffic 10:316-323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources