Control of macrophage activation and function by PPARs - PubMed (original) (raw)
Review
Control of macrophage activation and function by PPARs
Ajay Chawla. Circ Res. 2010.
Abstract
Abstract: Macrophages, a key component of the innate defense against pathogens, participate in the initiation and resolution of inflammation, and in the maintenance of tissues. These diverse and at times antithetical functions of macrophages are executed via distinct activation states, ranging from classical to alternative to deactivation. Because the dysregulation of macrophage activation is pathogenically linked to various metabolic, inflammatory and immune disorders, regulatory proteins controlling macrophage activation have emerged as important new therapeutic targets. Here, the mechanisms by which peroxisome proliferator-activated receptors (PPARs) transcriptionally regulate macrophage activation in health and disease states, including obesity, insulin resistance and cardiovascular disease, are reviewed.
Figures
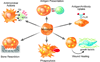
Figure 1. Functional heterogeneity in resident and recruited macrophages
Monocytic cells give rise to resident and recruited macrophages in tissues, which display tremendous functional heterogeneity. Clockwise from top left. Macrophages provide a first line of defense against intracellular pathogens by generating an inflammatory and respiratory burst and initiating antigen presentation to activate adaptive immunity; by clearing immune complexes and downregulating inflammatory responses; and by promoting wound healing via elaboration of growth factors. A subset of tissue macrophages, such as splenic red pulp macrophages and osteoclasts, become highly specialized in their location and function. The splenic red pulp macrophages are adapted for clearance and recycling of senescent red blood cells, whereas osteoclasts are critical for remodeling of bone throughout adult life.
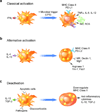
Figure 2. Macrophage activation contributes to functional heterogeneity
In response to cytokine signals, recruited and resident macrophages can undergo distinct activation programs. (a) Stimulation with the Th1 cytokine IFNγ and microbial byproducts, such as LPS, promotes maturation of classically activated macrophages. While the respiratory burst, reactive oxygen species (ROS) and nitric oxide (NO), promotes their microbicidal actions, secretion of pro-inflammatory cytokines, such as TNFα and IL-12, enhances cell mediated immunity. (b) Th2 cytokines IL-4 and IL-13 promote maturation of alternatively activated macrophages during infections with parasitic helminths. Although activated, as evidenced by induction of MHC class II and co-stimulatory molecules (PD-L2), these macrophages display a distinct repertoire of cell surface receptors (mannose receptor, Mrc1; dectin-1, Clec7a; and Mgl1, Clec10A), and secreted products (Ym-1, Chi3l3; and FIZZ1, Retnla). (c) In contrast, innate or acquired deactivation of macrophages by ingestion of apoptotic cells or by stimulation with IL-10 or glucocorticoids potently suppresses their capacity to present antigens and mount a pro-inflammatory burst. Unlike classical and alternative activation, deactivation dramatically suppresses the expression of MHC class II molecules. Abbreviations: IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; MHC, major histocompatability complex; MR, mannose receptor; NO, nitric oxide; PD-L, program death ligand; PPAR, peroxisome proliferator-activated receptor; ROS, reactive oxygen species; TGF-β, transforming growth factor-β; Th, T helper; TNF, tumor necrosis factor.
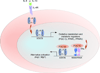
Figure 3. Cooperation between IL-4/STAT6 signaling and PPARs directs alternative macrophage activation
Stimulation of macrophages with the Th2 cytokines IL-4 and IL-13 leads to activation of JAKs (Janus associated kinases), resulting in tyrosine phosphorylation of STAT6. Phosphorylated STAT6 translocates to the nucleus where it activates the transcriptional program for alternative activation, including the induction of the nuclear receptors PPARγ and δ, and the coactivator protein PGC-1β. Cooperation among STAT6, PPARγ, and PGC-1β reprograms macrophages for oxidative metabolism by upregulating fatty acid oxidation and mitochondrial biogenesis, whereas transcriptional synergy between STAT6, PPARδ and PGC-1β is necessary for full expression of their immune phenotype.
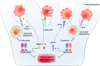
Figure 4. Nuclear receptors regulate macrophage deactivation
Two nuclear receptor signaling pathways are critical for macrophage deactivation after ingestion of apoptotic cells. Phagocytosis of apoptotic cells induces the expression of PPARδ in macrophages, which together with LXRs serve as transcriptional sensors of apoptotic cells. The degradation of apoptotic cells in lysosomes releases fatty acids and oxysterols, signaling molecules capable of transactivating PPARδ and LXRs, respectively. The transcriptional activation of these nuclear receptors enhances phagocytosis of apoptotic cells by inducing the expression of opsonins (C1q and Mfge8) and the cell surface receptor Mertk. In the presence of apoptotic cells, PPARδ and LXRs promote macrophage deactivation by enhancing the release of IL-10 and restraining the release of IL-12 and TNFα, leading to suppression of inflammation and autoimmunity.
Figure 5. Proposed mechanisms for inhibiting macrophage inflammation
Three distinct mechanisms have been identified to inhibit the inflammatory program of classically activated macrophages. (a) Activation of negative feedback loops results in inhibition of LPS and cytokine signaling. SOCS proteins, which are dramatically induced in activated macrophages, interfere with JAK-STAT and LPS signaling pathways. In contrast, ATF3 is a transcriptional activator that attenuates inflammation by preventing recruitment of NF-κB to its target genes. (b) SUMOlyated PPARγ inhibits macrophage inflammatory gene expression by blocking the release of NCoR corepressor complexes. Stimulation with TZDs promotes SUMOlyation of PPARγ in resting macrophages, resulting in inhibition of ubiquitin-mediated proteasomal degradation of NCoR repressor complexes from promoters of inflammatory genes. Consequently, failure to clear the NCoR corepressor checkpoint prevents transcriptional activation of inflammatory genes by LPS. (c) Sequestration of the transcriptional repressor BCL6 by PPARδ. Unliganded PPARδ interacts with BCL6, preventing it from repressing its target genes. Availability of PPARδ ligand or genetic deletion of PPARδ releases BCL6, allowing it to repress transcription of inflammatory genes.
Similar articles
- Mechanisms of macrophage activation in obesity-induced insulin resistance.
Odegaard JI, Chawla A. Odegaard JI, et al. Nat Clin Pract Endocrinol Metab. 2008 Nov;4(11):619-26. doi: 10.1038/ncpendmet0976. Epub 2008 Oct 7. Nat Clin Pract Endocrinol Metab. 2008. PMID: 18838972 Free PMC article. Review. - Inflammatory mediators and insulin resistance in obesity: role of nuclear receptor signaling in macrophages.
Fuentes L, Roszer T, Ricote M. Fuentes L, et al. Mediators Inflamm. 2010;2010:219583. doi: 10.1155/2010/219583. Epub 2010 May 20. Mediators Inflamm. 2010. PMID: 20508742 Free PMC article. Review. - [The role of PPARs and their isoforms in metabolic disorders related to insulin resistance and diabetes].
Kravchenko NA, Iarmysh NV. Kravchenko NA, et al. Tsitol Genet. 2011 May-Jun;45(3):68-78. Tsitol Genet. 2011. PMID: 21774406 Review. Russian. - PPARs: the vasculature, inflammation and hypertension.
Duan SZ, Usher MG, Mortensen RM. Duan SZ, et al. Curr Opin Nephrol Hypertens. 2009 Mar;18(2):128-33. doi: 10.1097/MNH.0b013e328325803b. Curr Opin Nephrol Hypertens. 2009. PMID: 19434050 Review. - Macrophages, inflammation, and insulin resistance.
Olefsky JM, Glass CK. Olefsky JM, et al. Annu Rev Physiol. 2010;72:219-46. doi: 10.1146/annurev-physiol-021909-135846. Annu Rev Physiol. 2010. PMID: 20148674 Review.
Cited by
- Transcriptome profiling of macrophages persistently infected with human respiratory syncytial virus and effect of recombinant Taenia solium calreticulin on immune-related genes.
Rivera-Toledo E, Fernández-Rojas MA, Santiago-Olivares C, Cruz-Rivera M, Hernández-Bautista V, Ávila-Horta F, Flisser A, Mendlovic F. Rivera-Toledo E, et al. Front Microbiol. 2024 Sep 4;15:1402589. doi: 10.3389/fmicb.2024.1402589. eCollection 2024. Front Microbiol. 2024. PMID: 39296294 Free PMC article. - Macrophage polarization and plasticity in health and disease.
Biswas SK, Chittezhath M, Shalova IN, Lim JY. Biswas SK, et al. Immunol Res. 2012 Sep;53(1-3):11-24. doi: 10.1007/s12026-012-8291-9. Immunol Res. 2012. PMID: 22418728 Review. - Exercise-induced increase in M2 macrophages accelerates wound healing in young mice.
Kawanishi M, Kami K, Nishimura Y, Minami K, Senba E, Umemoto Y, Kinoshita T, Tajima F. Kawanishi M, et al. Physiol Rep. 2022 Oct;10(19):e15447. doi: 10.14814/phy2.15447. Physiol Rep. 2022. PMID: 36200164 Free PMC article. - Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders.
Fukuda D, Aikawa E, Swirski FK, Novobrantseva TI, Kotelianski V, Gorgun CZ, Chudnovskiy A, Yamazaki H, Croce K, Weissleder R, Aster JC, Hotamisligil GS, Yagita H, Aikawa M. Fukuda D, et al. Proc Natl Acad Sci U S A. 2012 Jul 3;109(27):E1868-77. doi: 10.1073/pnas.1116889109. Epub 2012 Jun 13. Proc Natl Acad Sci U S A. 2012. PMID: 22699504 Free PMC article. - In obesity and weight loss, all roads lead to the mighty macrophage.
Red Eagle A, Chawla A. Red Eagle A, et al. J Clin Invest. 2010 Oct;120(10):3437-40. doi: 10.1172/JCI44721. Epub 2010 Sep 27. J Clin Invest. 2010. PMID: 20877005 Free PMC article.
References
- Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. - PubMed
- Evans RM, Barish GD, Wang Y-X. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. - PubMed
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. - PubMed
- Dreyer C, Keller H, Mahfoudi A, Laudet V, Krey G, Wahli W. Positive regulation of the peroxisomal beta-oxidation pathway by fatty acids through activation of peroxisome proliferator-activated receptors (PPAR) Biol Cell. 1993;77:67–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK062386/DK/NIDDK NIH HHS/United States
- R01 DK076760/DK/NIDDK NIH HHS/United States
- HL076746/HL/NHLBI NIH HHS/United States
- R01 DK081405/DK/NIDDK NIH HHS/United States
- R01 HL076746/HL/NHLBI NIH HHS/United States
- DP1 OD006415-01/OD/NIH HHS/United States
- K08 DK062386/DK/NIDDK NIH HHS/United States
- DP1 OD006415/OD/NIH HHS/United States
- R01 DK076760-03/DK/NIDDK NIH HHS/United States
- DK081405/DK/NIDDK NIH HHS/United States
- R01 HL076746-05/HL/NHLBI NIH HHS/United States
- R01 DK081405-02/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources