Severe iron deficiency blunts the response of the iron regulatory gene Hamp and pro-inflammatory cytokines to lipopolysaccharide - PubMed (original) (raw)
Severe iron deficiency blunts the response of the iron regulatory gene Hamp and pro-inflammatory cytokines to lipopolysaccharide
Deepak Darshan et al. Haematologica. 2010 Oct.
Abstract
Background: Expression of the key iron regulatory hormone hepcidin is increased by some stimuli (iron loading, inflammation) but decreased by others (increased erythropoiesis, iron deficiency). We investigated the response of hepcidin to increased erythropoiesis and iron deficiency in the presence of an acute inflammation to assess the relative strengths of these stimuli.
Design and methods: Sprague-Dawley rats were maintained on control or iron-deficient diets and treated with lipopolysaccharide to induce inflammation or phenylhydrazine to stimulate erythropoiesis. The levels of Hamp, IL-6 and α2m mRNA were determined by qualitative real-time polymerase chain reaction and those of serum interleukin-6 and tumor necrosis factor-α were measured by enzyme-linked immunosorbent assay. Cultured RAW264.7 and HuH7 cells were used in associated studies.
Results: The increase in hepatic hepcidin levels induced by lipopolysaccharide was not affected by phenylhydrazine treatment but was blunted by iron deficiency. Lipopolysaccharide-treated iron-deficient animals also showed lower liver α2m mRNA and reduced serum interleukin-6 and tumor necrosis factor-α, suggesting a more generalized effect of iron deficiency. Similarly, RAW 264.7 cells treated with iron chelators and then stimulated with lipopolysaccharide showed lower IL-6 mRNA than cells treated with lipopolysaccharide alone. Huh7 cells treated with an iron chelator showed a blunted hepcidin response to interleukin-6, suggesting that the response of hepatic parenchymal cells to inflammatory cytokines may also be iron-dependent.
Conclusions: In any one physiological situation, net hepcidin levels are determined by the relative strengths of competing stimuli. The ability of severe iron deficiency to blunt the response to lipopolysaccharide of both hepcidin and other markers of inflammation suggests that adequate iron levels are necessary for a full acute phase response.
Figures
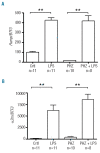
Figure 1.
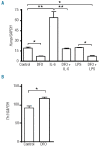
Hepcidin and α2m response to LPS is not affected by an increase in the rate of erythropoiesis. Expression of hepcidin (A) and α2m (B) mRNA was measured by quantitative real-time PCR in the livers of 9–10 week old female rats. Animals were treated with either LPS (0.1 mg/kg i.p.) or PHZ (100 mg/kg i.p.) or both 4 days and 6 h, respectively, prior to euthanasia. Data are presented as mean ± SEM and group sizes varied from 8–11 animals as indicated. **P<0.001.
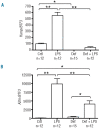
Figure 2.
Iron deficiency blunts the hepcidin and α2m response to LPS. Expression of hepcidin (A) and α2m (B) mRNA was measured by quantitative real-time PCR in the livers of 9- to 10-week old male rats. Animals were fed for 6 weeks with either an iron-replete diet (159 mg/kg) or iron-deficient diet (2–3 mg/kg)(to make them iron deficient) and then treated with LPS (0.1 mg/kg i.p.) 6 h prior to euthanasia. Data are presented as mean ± SEM and group sizes varied from 12–15 animals as indicated. *P<0.05 and **P<0.001.
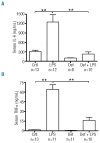
Figure 3.
Iron deficiency reduces cytokine levels in response to LPS treatment in rats. Serum IL-6 (A) and TNF-α (B) levels were determined by ELISA in male rats that were fed for 6 weeks with either an iron-replete diet (159 mg/kg) or iron-deficient diet (2–3 mg/kg) and then treated with LPS (0.1 mg/kg i.p.) 6 h prior to euthanasia. Data are presented as mean ± SEM and group sizes varied from 8–13 animals as indicated. **P<0.001.
Figure 4.
Iron chelation decreases the IL-6 level in cells in response to LPS treatment. IL-6 mRNA levels in RAW 264.7 cells were determined by quantitative real-time PCR after pretreatment with 20 μM desferrioxamine (DFO), 25 μM salicylaldehyde isonicotinoyl hydrazone (SIH) or medium alone for 6 h followed by overnight incubation with LPS (200 ng/mL). Treatments were carried out in triplicate and data are presented as mean ± SEM. **P<0.001. ND= none detected.
Figure 5.
Iron chelation reduces NFκB activation in primary hepatic macrophages. Freshly isolated rat primary hepatic macrophages (Küpffer cells) were treated for 6 h with 20 μM desferrioxamine (DFO) or medium alone then incubated overnight with LPS (200 ng/mL). Cell extracts were prepared as indicated in the Design and Methods section, subjected to polyacrylamide gel electrophoresis and western blotted for phospho-NFκB p65 and total NFκB p65. Triplicate samples are shown.
Figure 6.
Iron deficiency lowers IL-6 activity and increases TfR1 levels in liver-derived Huh7 cells. (A) Hepcidin mRNA levels were measured in Huh7 cells by quantitative real-time PCR. The cells were left untreated or treated with IL-6 (20 ng/mL) or LPS (200 ng/mL) for 6 h prior to harvest and analysis. Some cells were pretreated with desferrioxamine (DFO; 20 μM) for 6 h prior to IL-6 or LPS treatment. (B) Cells treated with DFO also showed an increase in TfR1 levels due to relative iron depletion. Treatments were carried out in triplicate and data are presented as mean ± SEM. *P<0.05 and **P<0.001.
Similar articles
- Low hepcidin accounts for the proinflammatory status associated with iron deficiency.
Pagani A, Nai A, Corna G, Bosurgi L, Rovere-Querini P, Camaschella C, Silvestri L. Pagani A, et al. Blood. 2011 Jul 21;118(3):736-46. doi: 10.1182/blood-2011-02-337212. Epub 2011 May 31. Blood. 2011. PMID: 21628413 - PGC-1α regulates hepatic hepcidin expression and iron homeostasis in response to inflammation.
Qian J, Chen S, Huang Y, Shi X, Liu C. Qian J, et al. Mol Endocrinol. 2013 Apr;27(4):683-92. doi: 10.1210/me.2012-1345. Epub 2013 Feb 25. Mol Endocrinol. 2013. PMID: 23438894 Free PMC article. - Iron metabolism in hepcidin1 knockout mice in response to phenylhydrazine-induced hemolysis.
Masaratana P, Latunde-Dada GO, Patel N, Simpson RJ, Vaulont S, McKie AT. Masaratana P, et al. Blood Cells Mol Dis. 2012 Aug 15;49(2):85-91. doi: 10.1016/j.bcmd.2012.04.003. Epub 2012 May 19. Blood Cells Mol Dis. 2012. PMID: 22609087 - Molecular mechanisms regulating hepcidin revealed by hepcidin disorders.
Camaschella C, Silvestri L. Camaschella C, et al. ScientificWorldJournal. 2011 Jul 7;11:1357-66. doi: 10.1100/tsw.2011.130. ScientificWorldJournal. 2011. PMID: 21789471 Free PMC article. Review. - Hepcidin--a peptide hormone at the interface of innate immunity and iron metabolism.
Ganz T. Ganz T. Curr Top Microbiol Immunol. 2006;306:183-98. doi: 10.1007/3-540-29916-5_7. Curr Top Microbiol Immunol. 2006. PMID: 16909922 Review.
Cited by
- Increased Hepcidin Levels During a Period of High Training Load Do Not Alter Iron Status in Male Elite Junior Rowers.
Zügel M, Treff G, Steinacker JM, Mayer B, Winkert K, Schumann U. Zügel M, et al. Front Physiol. 2020 Jan 21;10:1577. doi: 10.3389/fphys.2019.01577. eCollection 2019. Front Physiol. 2020. PMID: 32038278 Free PMC article. - Prevalence of Undernutrition and Anemia among Santal Adivasi Children, Birbhum District, West Bengal, India.
Stiller CK, Golembiewski SKE, Golembiewski M, Mondal S, Biesalski HK, Scherbaum V. Stiller CK, et al. Int J Environ Res Public Health. 2020 Jan 3;17(1):342. doi: 10.3390/ijerph17010342. Int J Environ Res Public Health. 2020. PMID: 31947849 Free PMC article. - Combinatorial effects of malaria season, iron deficiency, and inflammation determine plasma hepcidin concentration in African children.
Atkinson SH, Armitage AE, Khandwala S, Mwangi TW, Uyoga S, Bejon PA, Williams TN, Prentice AM, Drakesmith H. Atkinson SH, et al. Blood. 2014 May 22;123(21):3221-9. doi: 10.1182/blood-2013-10-533000. Epub 2014 Mar 4. Blood. 2014. PMID: 24596418 Free PMC article. - Circulating iron levels influence the regulation of hepcidin following stimulated erythropoiesis.
Mirciov CSG, Wilkins SJ, Hung GCC, Helman SL, Anderson GJ, Frazer DM. Mirciov CSG, et al. Haematologica. 2018 Oct;103(10):1616-1626. doi: 10.3324/haematol.2017.187245. Epub 2018 Jun 14. Haematologica. 2018. PMID: 29903760 Free PMC article. - The multicopper ferroxidase hephaestin enhances intestinal iron absorption in mice.
Fuqua BK, Lu Y, Darshan D, Frazer DM, Wilkins SJ, Wolkow N, Bell AG, Hsu J, Yu CC, Chen H, Dunaief JL, Anderson GJ, Vulpe CD. Fuqua BK, et al. PLoS One. 2014 Jun 4;9(6):e98792. doi: 10.1371/journal.pone.0098792. eCollection 2014. PLoS One. 2014. PMID: 24896847 Free PMC article.
References
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–3. - PubMed
- Frazer DM, Wilkins SJ, Becker EM, Vulpe CD, McKie AT, Trinder D, et al. Hepcidin expression inversely correlates with the expression of duodenal iron transporters and iron absorption in rats. Gastroenterology. 2002;123(3):835–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources