Equol: history, chemistry, and formation - PubMed (original) (raw)
Review
Equol: history, chemistry, and formation
Kenneth D R Setchell et al. J Nutr. 2010 Jul.
Abstract
Equol, first isolated from equine urine in 1932 and identified 50 years later in human urine as a metabolite of the soy isoflavones, daidzin and daidzein, is produced by intestinal bacteria in some, but not all, adults. This observation led to the term equol-producers to define those adults that could make equol in response to consuming soy isoflavones and the hypothesis that the health benefits of soy-based diets may be greater in equol-producers than in equol nonproducers. By virtue of a chiral center, equol occurs as a diastereoisomer and intestinal bacteria are enantiospecific in synthesizing exclusively the S-(-)equol enantiomer, an enantiomer that has selective affinity for the estrogen receptor-beta. Both enantiomers are of interest from a clinical and pharmacological perspective and are currently being developed as nutraceutical and pharmacological agents. The wide range of biological activities these enantiomers possess warrants their investigation for the treatment of a number of hormone-related conditions involving estrogen-dependent and androgen-related conditions. The following review describes the history, chemistry, and factors governing the intestinal bacterial formation of equol.
Figures
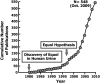
FIGURE 1
Cumulative number of publications on equol by year since its first identification in human urine.
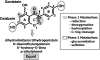
FIGURE 2
Principal metabolic biotransformations of the soy isoflavones daidzein and genistein. The structure for daidzein is shown and genistein has the identical structure but with an additional hydroxyl group at position C-5 of the A-ring, highlighted by the wavy arrow.
FIGURE 3
Differences in the type of soy foods consumed by Western and Asian populations may account for differences in the frequency of equol-producers.
Similar articles
- S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora.
Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, Handa RJ, Heubi JE. Setchell KD, et al. Am J Clin Nutr. 2005 May;81(5):1072-9. doi: 10.1093/ajcn/81.5.1072. Am J Clin Nutr. 2005. PMID: 15883431 Clinical Trial. - Equol: pharmacokinetics and biological actions.
Setchell KD, Clerici C. Setchell KD, et al. J Nutr. 2010 Jul;140(7):1363S-8S. doi: 10.3945/jn.109.119784. Epub 2010 Jun 2. J Nutr. 2010. PMID: 20519411 Free PMC article. Review. - The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers.
Setchell KD, Zhao X, Jha P, Heubi JE, Brown NM. Setchell KD, et al. Am J Clin Nutr. 2009 Oct;90(4):1029-37. doi: 10.3945/ajcn.2009.27981. Epub 2009 Aug 26. Am J Clin Nutr. 2009. PMID: 19710188 Free PMC article. Clinical Trial. - Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health.
Yuan JP, Wang JH, Liu X. Yuan JP, et al. Mol Nutr Food Res. 2007 Jul;51(7):765-81. doi: 10.1002/mnfr.200600262. Mol Nutr Food Res. 2007. PMID: 17579894 Review. - The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones.
Setchell KD, Brown NM, Lydeking-Olsen E. Setchell KD, et al. J Nutr. 2002 Dec;132(12):3577-84. doi: 10.1093/jn/132.12.3577. J Nutr. 2002. PMID: 12468591 Review.
Cited by
- Human Papillomavirus Infections and the Role Played by Cervical and Cervico-Vaginal Microbiota-Evidence from Next-Generation Sequencing Studies.
Głowienka-Stodolak M, Bagińska-Drabiuk K, Szubert S, Hennig EE, Horala A, Dąbrowska M, Micek M, Ciebiera M, Zeber-Lubecka N. Głowienka-Stodolak M, et al. Cancers (Basel). 2024 Jan 17;16(2):399. doi: 10.3390/cancers16020399. Cancers (Basel). 2024. PMID: 38254888 Free PMC article. Review. - Isoflavones for hypercholesterolaemia in adults.
Qin Y, Niu K, Zeng Y, Liu P, Yi L, Zhang T, Zhang QY, Zhu JD, Mi MT. Qin Y, et al. Cochrane Database Syst Rev. 2013 Jun 6;2013(6):CD009518. doi: 10.1002/14651858.CD009518.pub2. Cochrane Database Syst Rev. 2013. PMID: 23744562 Free PMC article. Review. - The gut microbiome as a modulator of arterial function and age-related arterial dysfunction.
Longtine AG, Greenberg NT, Bernaldo de Quirós Y, Brunt VE. Longtine AG, et al. Am J Physiol Heart Circ Physiol. 2024 Apr 1;326(4):H986-H1005. doi: 10.1152/ajpheart.00764.2023. Epub 2024 Feb 16. Am J Physiol Heart Circ Physiol. 2024. PMID: 38363212 Review. - Isolation and identification of an isoflavone reducing bacterium from feces from a pregnant horse.
Jinglong X, Xiaobin L, Fang Z, Chenchen W, Kailun Y. Jinglong X, et al. PLoS One. 2019 Nov 18;14(11):e0223503. doi: 10.1371/journal.pone.0223503. eCollection 2019. PLoS One. 2019. PMID: 31738752 Free PMC article. - A New Paradigm in the Relationship between Gut Microbiota and Breast Cancer: β-glucuronidase Enzyme Identified as Potential Therapeutic Target.
Fernández-Murga ML, Gil-Ortiz F, Serrano-García L, Llombart-Cussac A. Fernández-Murga ML, et al. Pathogens. 2023 Aug 26;12(9):1086. doi: 10.3390/pathogens12091086. Pathogens. 2023. PMID: 37764894 Free PMC article. Review.
References
- Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust J Agric Res. 1946;22:131–8. - PubMed
- Lightfoot RJ, Croker KP, Neil HG. Failure of sperm transport in relation to ewe infertility following prolonged grazing on oestrogenic pastures. Aust J Agric Res. 1968;18:755–65.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources