miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop - PubMed (original) (raw)
miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop
Kristina Kapinas et al. J Biol Chem. 2010.
Abstract
Differentiation of human mesenchymal stem cells into osteoblasts is controlled by extracellular cues. Canonical Wnt signaling is particularly important for maintenance of bone mass in humans. Post-transcriptional regulation of gene expression, mediated by microRNAs, plays an essential role in the control of osteoblast differentiation. Here, we find that miR-29a is necessary for human osteoblast differentiation, and miR-29a is increased during differentiation in the mesenchymal precursor cell line hFOB1.19 and in primary cultures of human osteoblasts. Furthermore, the promoter of the expressed sequence tag containing the human miR-29a gene is induced by canonical Wnt signaling. This effect is mediated, at least in part, by two T-cell factor/LEF-binding sites within the proximal promoter. Furthermore, we show that the negative regulators of Wnt signaling, Dikkopf-1 (Dkk1), Kremen2, and secreted frizzled related protein 2 (sFRP2), are direct targets of miR-29a. Endogenous protein levels for these Wnt antagonists are increased in cells transfected with synthetic miR-29a inhibitor. In contrast, transfection with miR-29a mimic decreases expression of these antagonists and potentiates Wnt signaling. Overall, we demonstrate that miR-29 and Wnt signaling are involved in a regulatory circuit that can modulate osteoblast differentiation. Specifically, canonical Wnt signaling induces miR-29a transcription. The subsequent down-regulation of key Wnt signaling antagonists, Dkk1, Kremen2, and sFRP2, by miR-29a potentiates Wnt signaling, contributing to a gene expression program important for osteoblast differentiation. This novel regulatory circuit provides additional insight into how microRNAs interact with signaling molecules during osteoblast differentiation, allowing for fine-tuning of intricate cellular processes.
Figures
FIGURE 1.
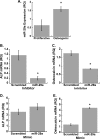
miR-29a is necessary for osteoblast differentiation. A, relative quantity of miR-29a normalized to 5 S rRNA. *, significantly different from proliferative conditions, p < 0.05. hFOB cells were transiently transfected with miR-29a inhibitor and then cultured for 3 days under osteoblast differentiation conditions. Relative quantity (RQ) of ALP (B) and osteocalcin mRNA expression (C) was normalized to 18 S rRNA. hFOB cells were transiently transfected with miR-29a mimic and then cultured for 3 days under osteoblast differentiation conditions. Relative quantity of ALP (D) and osteocalcin (E) mRNA expression was normalized to 18 S rRNA. *, significantly different from the scrambled negative control p < 0.05.
FIGURE 2.
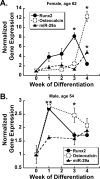
Characterization of miR-29a expression in primary cultures of human osteoblastic cells. Primary cells were cultured for up to 4 weeks post-confluence in osteoblast differentiation medium. Relative quantity of Runx2 (●), osteocalcin (○), and miR-29a (▴) RNA expression was normalized to 18 S or 5 S rRNA, respectively. A, 62-year-old female; B, 54-year-old male. *, significantly different from week 0 (confluence), p < 0.05.
FIGURE 3.
Canonical Wnt signaling induces miR-29a expression. A, activated (dephosphorylated) β-catenin protein normalized to total β-catenin protein in hFOB cells treated with 5–20 m
m
NaCl (control) or LiCl for 3 h. *, significantly different from NaCl, p < 0.05. B, relative luciferase activity (RLU) of TOPFlash/FOPFlash, in transiently transfected hFOB cells treated with 10 m
m
NaCl or LiCl for 6 h. *, significantly different from NaCl, p < 0.05. C, time course of miR-29a expression in response to 10 m
m
LiCl or NaCl. RQ = relative quantity normalized to 5 S rRNA. *, significantly different from NaCl, p < 0.05. D, induction of miR-29a expression in hFOB cells treated with 0–100 ng/ml recombinant human Wnt3a for 3 h. RQ = relative quantity normalized to 5 S rRNA. *, significantly different from 0 ng/ml Wnt3a (vehicle), p < 0.05. E, time course of miR-29a expression in hFOB cells treated with vehicle or 50 ng/ml Wnt3a for up to 6 h. RQ = relative quantity normalized to 5 S rRNA. *, significantly different from vehicle at that time point, p < 0.05.
FIGURE 4.
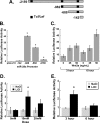
Canonical Wnt signaling induces miR-29a transcription. A, diagram of miR-29a promoter constructs, indicating the number of bases from the transcription start site. Predicted Tcf/Lef sites are represented by black bars. B, luciferase activity of the promoter constructs in transiently transfected hFOB cells. *, significantly different from pGL4.10 (vector alone), p < 0.05. C, activity of the −982 to −1 miR-29a promoter construct in transiently transfected hFOB cells treated with 0–50 ng/ml recombinant human Wnt3a for 3 or 6 h. *, significantly different from 0 ng/ml Wnt3a at that time point, p < 0.05. D, activity of the −982 to −1 miR-29a promoter construct in hFOB cells treated with 5–20 m
m
NaCl or LiCl for 3 h in hFOB cells. *, significantly different from NaCl, p < 0.05. E, activity of the −982 to −1 miR-29a promoter construct in hFOB cells treated with 10 m
m
NaCl or LiCl for 3 or 6 h in hFOB cells. *, significantly different from NaCl p < 0.05.
FIGURE 5.
Canonical Wnt signaling induces miR-29a promoter activity through two Tcf/Lef sites. A, diagram of the −982 to −1 miR-29a promoter and single or double mutants (mut) of the two predicted Tcf/Lef-binding sites. A black box indicates a wild type TCF/LEF site, and × indicates a mutated site. B, consensus sequences for the Tcf/Lef-binding site, the wild type sequences in the endogenous −982 to −1 miR-29a promoter, and the sequences of the mutated binding sites (underlined). Activity of wild type and mutant −982 to −1 base constructs in transiently transfected hFOB cells treated with 10 m
m
NaCl or LiCl for 3 h (C) or with vehicle or 50 ng/ml recombinant human Wnt3a for 6 h (D). *, significantly different from NaCl or vehicle p < 0.05; #, significantly different from WT/WT, p < 0.05.
FIGURE 6.
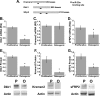
Wnt signaling antagonists are down-regulated during osteoblast differentiation. A, diagram of Kremen2, Dkk1, and sFRP2 3′-UTRs and potential miR-29a-binding sites. Relative quantity (RQ) of Dkk1 (B), Kremen2 (C), and sFRP2 (D) mRNA normalized to 18 S rRNA in hFOB cells. Relative expression of Dkk1 (E), Kremen2 (F), and sFRP2 (G) protein, normalized to actin, in hFOB cells. *, significantly different from proliferative conditions, p < 0.05. P, proliferative; O, osteogenic.
FIGURE 7.
miR-29a negatively regulates Wnt antagonists. A, activity of luciferase-3′-UTR constructs in transiently transfected hFOB cells, under proliferating or differentiating conditions. *, significantly different from proliferative conditions, p < 0.05. B, activity of luciferase-3′-UTR constructs in hFOB cells, transiently co-transfected with 70 nm miRNA inhibitor and cultured under differentiating conditions. *, significantly different from scrambled control. C, activity of luciferase-3′-UTR constructs in hFOB cells, transiently co-transfected with 70 nm miRNA mimic and cultured under proliferative conditions. *, significantly different from scrambled control p < 0.05. Protein levels for Dkk1 (D), Kremen2 (E), and sFRP2 (F) in differentiating hFOBs treated with 50–150 nm scrambled or miR-29a inhibitor. Protein expression was normalized to actin. *, significantly different from scrambled control, p < 0.05.
FIGURE 8.
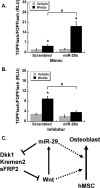
Wnt signaling is regulated by a miR-29/Wnt-positive feedback loop. Luciferase activity of TOPFlash/FOPFlash in hFOBs transiently co-transfected with 70 nm miRNA mimic (A) or 70 nm miRNA inhibitor (B), cultured in proliferating conditions. C, proposed model. Canonical Wnt signaling and miR-29 promote osteoblast differentiation through a variety of mechanisms (dotted lines). Canonical Wnt signaling induces miR-29a transcription. miR-29a subsequently down-regulates key Wnt signaling antagonists, Dkk1, Kremen2, and sFRP2, potentiating Wnt signaling. These two actions promote a gene expression program necessary for osteoblast differentiation.
Similar articles
- MicroRNA-29a ameliorates glucocorticoid-induced suppression of osteoblast differentiation by regulating β-catenin acetylation.
Ko JY, Chuang PC, Chen MW, Ke HC, Wu SL, Chang YH, Chen YS, Wang FS. Ko JY, et al. Bone. 2013 Dec;57(2):468-75. doi: 10.1016/j.bone.2013.09.019. Epub 2013 Oct 2. Bone. 2013. PMID: 24096265 - Essential role of nuclear factor of activated T cells (NFAT)-mediated Wnt signaling in osteoblast differentiation induced by strontium ranelate.
Fromigué O, Haÿ E, Barbara A, Marie PJ. Fromigué O, et al. J Biol Chem. 2010 Aug 13;285(33):25251-8. doi: 10.1074/jbc.M110.110502. Epub 2010 Jun 16. J Biol Chem. 2010. PMID: 20554534 Free PMC article. - Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma.
Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD Jr. Qiang YW, et al. Bone. 2008 Apr;42(4):669-80. doi: 10.1016/j.bone.2007.12.006. Epub 2007 Dec 27. Bone. 2008. PMID: 18294945 - Wnt signaling and cellular metabolism in osteoblasts.
Karner CM, Long F. Karner CM, et al. Cell Mol Life Sci. 2017 May;74(9):1649-1657. doi: 10.1007/s00018-016-2425-5. Epub 2016 Nov 26. Cell Mol Life Sci. 2017. PMID: 27888287 Free PMC article. Review. - Growth factor control of bone mass.
Canalis E. Canalis E. J Cell Biochem. 2009 Nov 1;108(4):769-77. doi: 10.1002/jcb.22322. J Cell Biochem. 2009. PMID: 19718659 Free PMC article. Review.
Cited by
- miR-375 regulates rat alveolar epithelial cell trans-differentiation by inhibiting Wnt/β-catenin pathway.
Wang Y, Huang C, Reddy Chintagari N, Bhaskaran M, Weng T, Guo Y, Xiao X, Liu L. Wang Y, et al. Nucleic Acids Res. 2013 Apr 1;41(6):3833-44. doi: 10.1093/nar/gks1460. Epub 2013 Feb 8. Nucleic Acids Res. 2013. PMID: 23396279 Free PMC article. - Protective effects of miR-29a on diabetic glomerular dysfunction by modulation of DKK1/Wnt/β-catenin signaling.
Hsu YC, Chang PJ, Ho C, Huang YT, Shih YH, Wang CJ, Lin CL. Hsu YC, et al. Sci Rep. 2016 Jul 27;6:30575. doi: 10.1038/srep30575. Sci Rep. 2016. PMID: 27460630 Free PMC article. - Pathogenesis of glucocorticoid-induced avascular necrosis: A microarray analysis of gene expression in vitro.
Bian Y, Qian W, Li H, Zhao RC, Shan WX, Weng X. Bian Y, et al. Int J Mol Med. 2015 Sep;36(3):678-84. doi: 10.3892/ijmm.2015.2273. Epub 2015 Jul 6. Int J Mol Med. 2015. PMID: 26151338 Free PMC article. - Regulation of Skeletal Homeostasis.
Zaidi M, Yuen T, Sun L, Rosen CJ. Zaidi M, et al. Endocr Rev. 2018 Oct 1;39(5):701-718. doi: 10.1210/er.2018-00050. Endocr Rev. 2018. PMID: 29897433 Free PMC article. Review. - Wnt signaling, a novel pathway regulating blood pressure? State of the art review.
Abou Ziki MD, Mani A. Abou Ziki MD, et al. Atherosclerosis. 2017 Jul;262:171-178. doi: 10.1016/j.atherosclerosis.2017.05.001. Epub 2017 May 4. Atherosclerosis. 2017. PMID: 28522145 Free PMC article. Review.
References
- Harris S. A., Enger R. J., Riggs B. L., Spelsberg T. C. (1995) J. Bone Miner. Res. 10, 178–186 - PubMed
- Friedenstein A., Kuralesova A. I. (1971) Transplantation 12, 99–108 - PubMed
- Phinney D. G., Prockop D. J. (2007) Stem Cells 25, 2896–2902 - PubMed
- Yen M. L., Chien C. C., Chiu I. M., Huang H. I., Chen Y. C., Hu H. I., Yen B. L. (2007) Stem Cells 25, 125–131 - PubMed
- Krause C., de Gorter D. J., Karperien M., ten Dijke P. (2008) Primer of the Metabolic Bone Diseases and Disorders of Mineral Metabolism (Rosen C., Compston J., Lian J. eds) 7th Ed., pp. 10–16, American Society of Bone and Mineral Research, Washington, D. C.
Publication types
MeSH terms
Substances
Grants and funding
- R56 AR044877/AR/NIAMS NIH HHS/United States
- AR44877/AR/NIAMS NIH HHS/United States
- R01 AR044877-13/AR/NIAMS NIH HHS/United States
- R01 AR044877/AR/NIAMS NIH HHS/United States
- R29 AR044877/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources