Activated networking of platelet activating factor receptor and FAK/STAT1 induces malignant potential in BRCA1-mutant at-risk ovarian epithelium - PubMed (original) (raw)
doi: 10.1186/1477-7827-8-74.
Dan Wang, Wei Jiang, Dale Edwards, Weiliang Qiu, Lisa M Barroilhet, Jung-Hyun Rho, Lianjin Jin, Vanitha Seethappan, Allison Vitonis, Jianliu Wang, Samuel C Mok, Christopher Crum, Daniel W Cramer, Bin Ye
Affiliations
- PMID: 20576130
- PMCID: PMC2903602
- DOI: 10.1186/1477-7827-8-74
Activated networking of platelet activating factor receptor and FAK/STAT1 induces malignant potential in BRCA1-mutant at-risk ovarian epithelium
Lifang Zhang et al. Reprod Biol Endocrinol. 2010.
Abstract
Objectives: It is essential to understand the molecular basis of ovarian cancer etiology and tumor development to provide more effective preventive and therapeutic approaches to reduce mortality. Particularly, the molecular targets and pathways involved in early malignant transformation are still not clear. Pro-inflammatory lipids and pathways have been reported to play significant roles in ovarian cancer progression and metastasis. The major objective of this study was to explore and determine whether platelet activating factor (PAF) and receptor associated networking pathways might significantly induce malignant potential in BRCA1-mutant at-risk epithelial cells.
Methods: BRCA1-mutant ovarian epithelial cell lines including (HOSE-636, HOSE-642), BRCA1-mutant ovarian cancer cell (UWB1.289), wild type normal ovarian epithelial cell (HOSE-E6E7) and cancerous cell line (OVCA429), and the non-malignant BRCA1-mutant distal fallopian tube (fimbria) tissue specimens were used in this study. Mutation analysis, kinase microarray, western blot, immune staining, co-immune precipitation, cell cycle, apoptosis, proliferation and bioinformatic pathway analysis were applied.
Results: We found that PAF, as a potent pro-inflammatory mediator, induced significant anti-apoptotic effect in BRCA1-mutant ovarian surface epithelial cells, but not in wild type HOSE cells. With kinase microarray technology and the specific immune approaches, we found that phosphor-STAT1 was activated by 100 nM PAF treatment only in BRCA1-mutant associated at-risk ovarian epithelial cells and ovarian cancer cells, but not in BRCA1-wild type normal (HOSE-E6E7) or malignant (OVCA429) ovarian epithelial cells. Co-immune precipitation revealed that elevated PAFR expression is associated with protein-protein interactions of PAFR-FAK and FAK-STAT1 in BRCA1-mutant ovarian epithelial cells, but not in the wild-type control cells.
Conclusion: Previous studies showed that potent inflammatory lipid mediators such as PAF and its receptor (PAFR) significantly contribute to cancer progression and metastasis. Our findings suggest that these potent inflammatory lipids and receptor pathways are significantly involved in the early malignant transformation through PAFR-FAK-STAT1 networking and to block apoptosis pathway in BRCA1 dysfunctional at-risk ovarian epithelium.
Figures
Figure 1
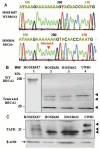
Molecular characteristics of the BRCA1 mutation in (BRCA1+) ovarian epithelial cells. (A) DNA extracted from the HOSE-636 and HOSE-E6E7 cells were subjected to BRCA1 gene sequence analysis with the standard protocol. 1961 delA mutation was detected in HOSE-636 cells. (B) Total protein lysis (30 μg) of Western blot showed pieces of mutant fragments of BRCA1 protein in HOSE-E6E7, HOSE-636 HOSE-642, and UWB1 cells were applied on SDS-PAGE and western blot analysis. Intact BRCA1 protein and its fragments were revealed by BRCA1 antibody. The separated western blots are required because of the nature and working conditions are different between BRCA1 intact protein and the truncated peptides. (C) PAFR protein was detected by western blot in wild type HOSE-E6E7 and BRCA1-mutant HOSE-636, HOSE-642, and UWB1 cell lines with a specific polyclonal antibody against human PAFR. The equal protein loading was normalized by β-actin.
Figure 2
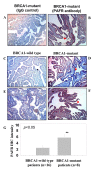
Immune staining of PAFR expression in epithelial cells of fallopian tube fimbria of BRCA1+ patients. (A, B) Immune staining of epithelial cells of fallopian tube fimbria of BRCA1+ patients with and without PAFR antibody. (C, E) Negative PAFR staining on the BRCA1 wild type epithelial cells of fallopian tube fimbria. (D, F) Positive immune staining of PAFR in epithelial cells of fallopian tube fimbria of the BRCA1+ patients. (G) Summary of the intensity scale (mean value with standard error bar) of immune histochemistry staining of the epithelial cells of fallopian tube collected from the patients without (n = 16) and with BRCA1-muation (n = 8) (p < 0.05). The grading system (1-10) of the immune intensity was used for semi-quantification and same set of slides were read by two independent investigators.
Figure 3
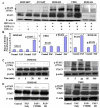
Bioinformatic pathway and protein-protein interaction between PAFR-BRCA1. (A). Pathway Studio software (Ariadne Inc.) and Human ResNet Mammalian Database were used in the study. Antibody microarray based protein kinase profiling and identification of STAT1 and STAT4 in BRCA1-mutant and PAFR-over expression (PAFR+) ovarian epithelial cancer cells. UWB1 cells were treated with PAF (100 nM) for 24 hours. Images of kinase arrays of signals after the incubation with 310 μg protein lysate of UWB1 cells treated with 100 nM PAF only (B) or with combination of 100 μM ginkgolide B (GB) and 100 nM PAF (C). The duplicated internal positive controls were used to calibrate the detection signal of membranes of the treated and untreated experiments. The summary of kinase expression profile of the UWB1 cells with different treatments and significance comparison by the paired student t-test (D).
Figure 4
Activated phospho-STAT1 in BRCA1 + ovarian epithelial cells. (A) Wild type HOSE-E6E7 and OVCA429 cancer cells and _BRCA1_-mutant at-risk (HOSE-642, HOSE-636) cells and _BRCA1_-mutant ovarian cancer cells (UWB1) were subjected to PAF treatment for 20 minutes and phospho-STAT1 (Y701) detection by western blot. (B) Summary of intensity of SATA1 and phosphor-STAT1 induced by PAF treatment in HOSE-642, UWB1 and HOSE-636 cells. Phosphor-STAT1 was significantly (p < 0.01) increased in all BRCA1-mutant cells. (C) Activation of phosphor-STAT1 at different time points (0, 5, 20 60 min and 360 min) of PAF treatment (100 nM) in _BRCA1_-mutant HOSE-642 and UWB1 cells. (D) Equal amount of protein lysate of the cells of untreated, treated with PAF (100 nM) alone or in combination of PAF and PAFR-inhibitor (10 μM) and GB (100 μM) were applied in western blot detection. DMSO and pure IgG (not shown) were used as control treatment for 20 minutes in both HOSE-642 and UWB1 cells. Equal loading of protein was calibrated with expression of β-actin.
Figure 5
PAF induced cell proliferation and anti-apoptosis in BRCA1-mutant ovarian epithelial cells. (A) The induced cell proliferation pattern in wild type HOSE-E6E7 and HOSE-27 cells and _BRCA1-_mutant HOSE-642 and HOSE-636 and ovarian malignant cells (UWB1) was affected by 72 hours treatment with different concentrations of PAF. Significant increase or decrease of cell proliferation induced by PAF treatment was indicated by symbol star (* p < 0.05). (B) Differential cell proliferation pattern was affected by PAF (1 nM) treatment in wild type of HOSE-E6E7 and HOSE-27 cells and _BRCA1_-mutant non-malignant HOSE-642 and HOSE-636 and ovarian cancer cells (UWB1), compared to the control cells without PAF treatment (as 100%). (C) PAFR-inhibitor, specific PAFR antibody and different concentration of antagonist ginkgolide B (1, 5, 10, 50, 100 μM) significantly (p < 0.05) blocked the PAF-induced proliferation in three _BRCA1_-mutant ovarian epithelial cell lines, compared to the control cells treated either with equal volume of DMSO or with IgG (as 100%). (D, E) Anti-apoptotic activity was significantly (p < 0.05) induced by PAF treatment in _BRCA1_-mutant at-risk HOSE-642 cells. (F, G) Apoptosis was significantly induced by PAF (100 nM) in wild-type HOSE-E6E7 cells, compared to the controls with 72 h treatment of equal volume DMSO. Experiments were performed at least three times (p < 0.05).
Figure 6
Activation of phosphor-FAK and STAT1 is associated with PAFR in BRCA1 mutant ovarian epithelial cells. (A) The specific phosphorylation of FAK in _BRCA1_-mutant at-risk HOSE-642 and HOSE-636 cells but not in wild type HOSE-E6E7 cells. (B) Summary of total FAK protein and phosphor-FAK protein induced by PAF treatment in wild type and BRCA1-mutant ovarian epithelial cells. The significant increases (*p < 0.05, **p < 0.01) were compared to the control cells treated with equal volume of DMSO (as 100%). (C) Protein-protein interaction between PAFR and FAK was detected by co-immune precipitation. Antibodies against PAFR and FAK were used for immune precipitation and incubated with protein lysate of HOSE-642 cells with 10 nm PAF treatment, and non-specific IgG was used as negative control. The immune precipitated protein complex was separated on SDS gels and detected by FAK and PAFR antibodies, respectively. (D). Protein-protein interaction between FAK and STAT1 was detected either by immune depletion assay or by co-immune precipitation with phosphor- and non-phospho antibodies of STAT1 and FAK. (E) Simplified scheme to show the integrative signal cascade and activation of PAFR, FAK, and STAT1 as early events of malignant transformation in BRCA1-mutant at-risk ovarian epithelium, particularly under the inflammatory conditions mediated lipid signaling (i.e. PAF).
Similar articles
- The Platelet-Activating Factor Receptor's Association with the Outcome of Ovarian Cancer Patients and Its Experimental Inhibition by Rupatadine.
Deuster E, Hysenaj I, Kahaly M, Schmoeckel E, Mayr D, Beyer S, Kolben T, Hester A, Kraus F, Chelariu-Raicu A, Burges A, Mahner S, Jeschke U, Trillsch F, Czogalla B. Deuster E, et al. Cells. 2021 Sep 7;10(9):2337. doi: 10.3390/cells10092337. Cells. 2021. PMID: 34571986 Free PMC article. - Transactivation of epidermal growth factor receptor through platelet-activating factor/receptor in ovarian cancer cells.
Yu Y, Zhang M, Zhang X, Cai Q, Zhu Z, Jiang W, Xu C. Yu Y, et al. J Exp Clin Cancer Res. 2014 Sep 28;33(1):85. doi: 10.1186/s13046-014-0085-6. J Exp Clin Cancer Res. 2014. PMID: 25261977 Free PMC article. - Activation of platelet-activating factor receptor and pleiotropic effects on tyrosine phospho-EGFR/Src/FAK/paxillin in ovarian cancer.
Aponte M, Jiang W, Lakkis M, Li MJ, Edwards D, Albitar L, Vitonis A, Mok SC, Cramer DW, Ye B. Aponte M, et al. Cancer Res. 2008 Jul 15;68(14):5839-48. doi: 10.1158/0008-5472.CAN-07-5771. Cancer Res. 2008. PMID: 18632638 Free PMC article. - Ovarian surface epithelium: family history and early events in ovarian cancer.
Wong AS, Auersperg N. Wong AS, et al. Reprod Biol Endocrinol. 2003 Oct 7;1:70. doi: 10.1186/1477-7827-1-70. Reprod Biol Endocrinol. 2003. PMID: 14609432 Free PMC article. Review. - Cells of origin of ovarian cancer: ovarian surface epithelium or fallopian tube?
Klotz DM, Wimberger P. Klotz DM, et al. Arch Gynecol Obstet. 2017 Dec;296(6):1055-1062. doi: 10.1007/s00404-017-4529-z. Epub 2017 Sep 23. Arch Gynecol Obstet. 2017. PMID: 28940023 Review.
Cited by
- PLA2G7/PAF-AH as Potential Negative Regulator of the Wnt Signaling Pathway Mediates Protective Effects in BRCA1 Mutant Breast Cancer.
Liao Y, Badmann S, Kraus F, Topalov NE, Mayr D, Kolben T, Hester A, Beyer S, Mahner S, Jeschke U, Trillsch F, Czogalla B, Burges A. Liao Y, et al. Int J Mol Sci. 2023 Jan 3;24(1):882. doi: 10.3390/ijms24010882. Int J Mol Sci. 2023. PMID: 36614323 Free PMC article. - Gene Expression Profiling in Ovaries and Association Analyses Reveal HEP21 as a Candidate Gene for Sexual Maturity in Chickens.
Chen B, Liang G, Zhu X, Tan Y, Xu J, Wu H, Mao H, Zhang Y, Chen J, Rao Y, Zhou M, Liu S. Chen B, et al. Animals (Basel). 2020 Jan 21;10(2):181. doi: 10.3390/ani10020181. Animals (Basel). 2020. PMID: 31973127 Free PMC article. - Epidermal growth factor induces platelet-activating factor production through receptors transactivation and cytosolic phospholipase A2 in ovarian cancer cells.
Yu Y, Zhang X, Hong S, Zhang M, Cai Q, Jiang W, Xu C. Yu Y, et al. J Ovarian Res. 2014 Apr 11;7:39. doi: 10.1186/1757-2215-7-39. eCollection 2014. J Ovarian Res. 2014. PMID: 24721622 Free PMC article. - The Platelet-Activating Factor Receptor's Association with the Outcome of Ovarian Cancer Patients and Its Experimental Inhibition by Rupatadine.
Deuster E, Hysenaj I, Kahaly M, Schmoeckel E, Mayr D, Beyer S, Kolben T, Hester A, Kraus F, Chelariu-Raicu A, Burges A, Mahner S, Jeschke U, Trillsch F, Czogalla B. Deuster E, et al. Cells. 2021 Sep 7;10(9):2337. doi: 10.3390/cells10092337. Cells. 2021. PMID: 34571986 Free PMC article. - Transactivation of epidermal growth factor receptor through platelet-activating factor/receptor in ovarian cancer cells.
Yu Y, Zhang M, Zhang X, Cai Q, Zhu Z, Jiang W, Xu C. Yu Y, et al. J Exp Clin Cancer Res. 2014 Sep 28;33(1):85. doi: 10.1186/s13046-014-0085-6. J Exp Clin Cancer Res. 2014. PMID: 25261977 Free PMC article.
References
- Fedorova OE, Liubchenko LN, Paiadini Iu G, Kazubskaia TP, Amosenko FA, Gar'kavtseva RF, Zasedatelev AS, Nasedkina TV. [Analysis of BRCA1/2 and CHEK2 mutations in ovarian cancer and primary multiple tumors involving the ovaries. Patients of Russian population using biochips] Mol Biol (Mosk) 2007;41:37–42. doi: 10.1134/S0026893307010050. - DOI - PubMed
- Berchuck A, Heron KA, Carney ME, Lancaster JM, Fraser EG, Vinson VL, Deffenbaugh AM, Miron A, Marks JR, Futreal PA, Frank TS. Frequency of germline and somatic BRCA1 mutations in ovarian cancer. Clin Cancer Res. 1998;4:2433–2437. - PubMed
- Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, LaPolla J, Hoffman M, Martino MA, Wakeley K, Wilbanks G, Nicosia S, Cantor A, Sutphen R. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–2816. doi: 10.1002/cncr.21536. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous