Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling - PubMed (original) (raw)
Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling
John Maciejowski et al. J Cell Biol. 2010.
Abstract
The spindle assembly checkpoint (SAC) in mammals uses cytosolic and kinetochore-based signaling pathways to inhibit anaphase. In this study, we use chemical genetics to show that the protein kinase Mps1 regulates both aspects of the SAC. Human MPS1-null cells were generated via gene targeting and reconstituted with either the wild-type kinase (Mps1(wt)) or a mutant version (Mps1(as)) sensitized to bulky purine analogues. Mps1 inhibition sharply accelerated anaphase onset, such that cells completed mitosis in 12 min, and prevented Cdc20's association with either Mad2 or BubR1 during interphase, i.e., before the appearance of functional kinetochores. Furthermore, intramitotic Mps1 inhibition evicted Bub1 and all other known SAC transducers from the outer kinetochore, but contrary to a recent study, did not perturb aurora B-dependent phosphorylation. We conclude that Mps1 has two complementary roles in SAC regulation: (1) initial cytoplasmic activation of Cdc20 inhibitors and (2) recruitment of factors that promote sustained anaphase inhibition and chromosome biorientation to unattached kinetochores.
Figures
Figure 1.
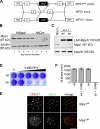
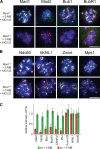
Generation of Mps1 conditional–null and analogue-sensitive human cells. (A) Schematic of AAV vectors used to mutate the MPS1 locus. Circles and triangles denote FRT and loxP sites, respectively. ITR, inverted terminal repeat. (B) _MPS1_flox/Δ cells were infected with the indicated adenoviruses and analyzed for Mps1 expression by immunoblotting. (C) _MPS1_flox/Δ and _MPS1_Δ/Δ cells complemented by wild-type (wt) or analogue-sensitive (as) Mps1 transgenes were analyzed by immunoblotting. LAP, localization and affinity purification. (D) Allele-specific inhibition of Mps1as. Cells were cultured in the presence of the bulky purine analogue 3MB-PP1 (from left to right: 0, 0.078, 0.313, 1.25, and 5 µM) for 7 d and stained with crystal violet. (E) Cells were fixed and stained with antibodies to centromere autoantigens (CREST; red) and transgene-encoded Mps1wt and Mps1as (green). Bar, 10 µm. (F) Mps1as inhibition overrides the SAC. Cells were filmed at 10-min intervals during treatment with nocodazole in the presence or absence of 10 µM 3MB-PP1 to assess the length of M phase (defined as the period of cell rounding by phase-contrast microscopy). Error bars indicate SEM.
Figure 2.
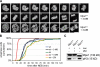
Mps1 is a component of the M phase timer. (A) Mps1wt and Mps1as cells stably expressing mCherry-tagged histone H2B were treated with 10 µM 3MB-PP1 and filmed at 1-min intervals by spinning-disk confocal microscopy. Maximum intensity projections are shown (
Videos 1–6
). Bar, 10 µm. (B) Quantification of the mitotic timing defect in Mps1-inhibited cells. Cumulative frequency of anaphase onset is plotted as a function of time after NEB. (C) Strong aurora B inhibition does not block the autophosphorylation of Mps1. HEK293 cells were transfected with GFP-tagged Mps1 expression plasmids, arrested in M phase with nocodazole (noc), and treated with or without 2 µM ZM for 2 h. Cell extracts were resolved by SDS-PAGE and probed with antibodies to serine 10–phosphorylated histone H3 (to confirm suppression of aurora B kinase activity) and GFP (to assess Mps1’s electrophoretic mobility, which becomes retarded by its mitotic activation and autophosphorylation; Stucke et al., 2004).
Figure 3.
Mps1 directs the assembly of Cdc20 inhibitory complexes in interphase. (A) Mps1wt and Mps1as cells were treated with 3MB-PP1 for 2 h. Extracts were immunoprecipitated with antibodies to Cdc20 and resolved by SDS-PAGE. Levels of BubR1, APC8, Mad2, and Cdc20 in each IP were determined by quantitative immunoblotting. The ratio of each protein to Cdc20 was computed and normalized to ratios obtained from untreated Mps1wt cells (= 1.0). (B) Mps1wt cells were treated with ZM for 2 h and processed as in A. White line indicates that intervening lanes have been spliced out. (C) Interphase and mitotic Cdc20 IPs were electrophoresed on 6% gels to resolve mitotically phosphorylated APC8 (left) and BubR1 (right) polypeptides as distinct species. Numbers at the bottom are loading amounts. Black lines indicate that intervening lanes have been spliced out. ctrl, control. Error bars indicate SEM.
Figure 4.
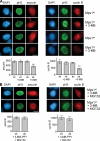
Mps1 protects cyclin B and securin from premature destruction. (A) Cells of the indicated genotypes were treated with or without 3MB-PP1, fixed, and stained with antibodies to serine 10–phosphorylated histone H3 (pH3) and securin (left) or cyclin B (right). Nuclei were counterstained with DAPI. Prophase cells were identified by their uniform pH3 staining (Hendzel et al., 1997), partially condensed chromatin (left), and nuclear import of cyclin B (right; Hagting et al., 1999). Note that cyclin B staining used methanol fixation, which preserves chromatin condensation less well than aldehyde fixation. (B) Cells were treated with both 3MB-PP1 and MG132 for 2 h and analyzed as in A. Asterisks indicate statistically significant deviation (P < 0.01 by one-way analysis of variance) from Mps1wt cells treated with 3MB-PP1. Error bars indicate SEM. Bars, 10 µm.
Figure 5.
Mps1 continuously stabilizes Cdc20 inhibitory complexes in M phase. (A) Mitotic cells were harvested by STLC treatment and shake off and incubated in medium containing STLC, 3MB-PP1, and/or MG132 for 2 h. Extracts were immunoprecipitated with antibodies to Cdc20 and resolved by SDS-PAGE. Levels of BubR1, APC8, Mad2, and Cdc20 in each IP were determined by quantitative immunoblotting. The ratio of each protein to Cdc20 was computed and normalized to ratios obtained from STLC-treated Mps1wt cells (= 1.0). (B) Extracts were analyzed as in A except that Mad2 antibodies were used for IP, and Mad1 antibodies were also used for immunoblotting. (C) Cells were arrested in M phase via overnight treatment with nocodazole. At time 0, cells were either treated with 3MB-PP1 or left untreated as a control. Samples were collected at 30-min intervals for analysis of cyclin B levels (left) and determination of mitotic indices (right). Black lines indicate that intervening lanes have been spliced out. Error bars indicate SEM.
Figure 6.
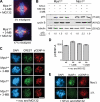
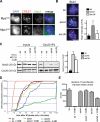
Mps1 promotes chromosome biorientation independently of aurora B. (A) Cells of the indicated genotypes were treated with 3MB-PP1 and MG132 for 1 h, fixed, and stained to detect centromeres (CREST; green), spindle MTs (α-tubulin; red), and chromosomes (DAPI; blue). (B) Mitotic Mps1wt and Mps1as cells were collected via STLC treatment and shake off and maintained in the presence of STLC, MG132, and/or 3MB-PP1 for 2 h. Lysates were resolved by SDS-PAGE and quantitative immunoblotting. The ratio of phosphorylated histone H3 (pH3) to tubulin was computed for each lane and normalized to the ratio in STLC-treated Mps1wt cells. (C) Cells were treated with nocodazole (noc) for 4 h to activate the SAC and treated for an additional 4 h with nocodazole, MG132, and/or 3MB-PP1. Centromeres (CREST) and serine 7–phosphorylated CENP-A (pCENP-A) were detected by immunofluorescence microscopy. (D) Quantification of results in C is shown. At least 100 centromeres in at least five cells were scored per sample. Error bars indicate SD. (E) _MPS1_flox/Δ cells were infected with Adβgal (top) or AdCre (bottom). 3 d later, both populations were treated with STLC and MG132 for 30 min, fixed, and stained with the indicated antibodies. BubR1 loss from kinetochores was used as a functional marker of Mps1 inactivation (Fig. 7). Bars, 10 µm.
Figure 7.
Mps1 kinase activity is continuously required to maintain Bub1 and all other SAC effectors at unattached kinetochores. (A and B) Mps1wt and Mps1as cells were treated with nocodazole for 4 h to activate the SAC and treated with nocodazole, MG132, and/or 3MB-PP1 for an additional 2 h. Cells were fixed and stained with antibodies against the indicated SAC or kinetochore components (green) and CREST antiserum (red). Note that cells not treated with 3MB-PP1 retained normal kinetochore localization patterns and, thus, have been omitted from these montages for clarity. Additional kinetochore/centromere proteins analyzed in this assay are shown in
Fig. S2
. (C) Kinetochore (KT)-specific signal intensities were determined in 3MB-PP1–treated Mps1as and Mps1wt cells (>100 kinetochores in more than five cells per sample) and normalized to the equivalent values in untreated Mps1wt cells. Error bars indicate SEM. Bars, 10 µm.
Figure 8.
Cytosolic-specific rescue of Mps1 inhibition restores mitotic timing and SAC proficiency to human cells. (A) Cells were fixed and stained with antibodies to centromere autoantigens (CREST; red) and transgene-encoded Mps1wt and Mps1ΔN (green). (B) Mps1as, Mps1as/wt, and Mps1as/ΔN cells were treated with nocodazole for 4 h to activate the SAC and treated with nocodazole, MG132, and/or 3MB-PP1 for an additional 2 h. Cells were fixed and stained with antibodies against Bub1 (green) and CREST antiserum (red). Kinetochore (KT)-specific signal intensities were determined in 3MB-PP1–treated Mps1as, Mps1as/wt, and Mps1as/ΔN cells (>100 kinetochores in more than five cells per sample) and normalized to values in untreated Mps1as cells. Bar, 10 µm. (C) Mitotic cells were harvested by overnight STLC treatment and shake off and incubated in medium containing STLC, 3MB-PP1, and/or MG132 for 2 h. Extracts and Cdc20 immunoprecipitates were analyzed as in Fig. 5. Recovery of Mad2 relative to Cdc20 was determined and normalized to its recovery from STLC- and MG132-treated Mps1as cells (= 1.0). Black lines indicate that intervening lanes have been spliced out. (D) Quantification of mitotic timing is shown. Cumulative frequency of anaphase onset is plotted as a function of time after NEB. (E) Mps1ΔN rescues the SAC. Cells were traced by phase-contrast microscopy during treatment with nocodazole ± 3MB-PP1 (compare with Fig. 1 F). Error bars indicate SEM.
Similar articles
- Monopolar spindle 1 (MPS1) kinase promotes production of closed MAD2 (C-MAD2) conformer and assembly of the mitotic checkpoint complex.
Tipton AR, Ji W, Sturt-Gillespie B, Bekier ME 2nd, Wang K, Taylor WR, Liu ST. Tipton AR, et al. J Biol Chem. 2013 Dec 6;288(49):35149-58. doi: 10.1074/jbc.M113.522375. Epub 2013 Oct 22. J Biol Chem. 2013. PMID: 24151075 Free PMC article. - Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex.
Hewitt L, Tighe A, Santaguida S, White AM, Jones CD, Musacchio A, Green S, Taylor SS. Hewitt L, et al. J Cell Biol. 2010 Jul 12;190(1):25-34. doi: 10.1083/jcb.201002133. J Cell Biol. 2010. PMID: 20624899 Free PMC article. - Spindle checkpoint protein dynamics at kinetochores in living cells.
Howell BJ, Moree B, Farrar EM, Stewart S, Fang G, Salmon ED. Howell BJ, et al. Curr Biol. 2004 Jun 8;14(11):953-64. doi: 10.1016/j.cub.2004.05.053. Curr Biol. 2004. PMID: 15182668 - The mitotic checkpoint: a signaling pathway that allows a single unattached kinetochore to inhibit mitotic exit.
Chan GK, Yen TJ. Chan GK, et al. Prog Cell Cycle Res. 2003;5:431-9. Prog Cell Cycle Res. 2003. PMID: 14593737 Review.
Cited by
- A novel mutation in the N-terminal domain of Drosophila BubR1 affects the spindle assembly checkpoint function of BubR1.
Duranteau M, Montagne JJ, Rahmani Z. Duranteau M, et al. Biol Open. 2016 Nov 15;5(11):1674-1679. doi: 10.1242/bio.021196. Biol Open. 2016. PMID: 27742609 Free PMC article. - How oocytes try to get it right: spindle checkpoint control in meiosis.
Touati SA, Wassmann K. Touati SA, et al. Chromosoma. 2016 Jun;125(2):321-35. doi: 10.1007/s00412-015-0536-7. Epub 2015 Aug 11. Chromosoma. 2016. PMID: 26255654 Review. - Leader of the SAC: molecular mechanisms of Mps1/TTK regulation in mitosis.
Pachis ST, Kops GJPL. Pachis ST, et al. Open Biol. 2018 Aug;8(8):180109. doi: 10.1098/rsob.180109. Open Biol. 2018. PMID: 30111590 Free PMC article. Review. - Effects of the selective MPS1 inhibitor MPS1-IN-3 on glioblastoma sensitivity to antimitotic drugs.
Tannous BA, Kerami M, Van der Stoop PM, Kwiatkowski N, Wang J, Zhou W, Kessler AF, Lewandrowski G, Hiddingh L, Sol N, Lagerweij T, Wedekind L, Niers JM, Barazas M, Nilsson RJ, Geerts D, De Witt Hamer PC, Hagemann C, Vandertop WP, Van Tellingen O, Noske DP, Gray NS, Würdinger T. Tannous BA, et al. J Natl Cancer Inst. 2013 Sep 4;105(17):1322-31. doi: 10.1093/jnci/djt168. Epub 2013 Aug 12. J Natl Cancer Inst. 2013. PMID: 23940287 Free PMC article. - Haspin inhibitors reveal centromeric functions of Aurora B in chromosome segregation.
Wang F, Ulyanova NP, Daum JR, Patnaik D, Kateneva AV, Gorbsky GJ, Higgins JM. Wang F, et al. J Cell Biol. 2012 Oct 15;199(2):251-68. doi: 10.1083/jcb.201205106. J Cell Biol. 2012. PMID: 23071152 Free PMC article.
References
- Burkard M.E., Randall C.L., Larochelle S., Zhang C., Shokat K.M., Fisher R.P., Jallepalli P.V. 2007. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc. Natl. Acad. Sci. USA. 104:4383–4388 10.1073/pnas.0701140104 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources