Mitochondrial loss, dysfunction and altered dynamics in Huntington's disease - PubMed (original) (raw)
. 2010 Oct 15;19(20):3919-35.
doi: 10.1093/hmg/ddq306. Epub 2010 Jul 21.
Affiliations
- PMID: 20660112
- PMCID: PMC2947400
- DOI: 10.1093/hmg/ddq306
Mitochondrial loss, dysfunction and altered dynamics in Huntington's disease
Jinho Kim et al. Hum Mol Genet. 2010.
Abstract
Although a direct causative pathway from the gene mutation to the selective neostriatal neurodegeneration remains unclear in Huntington's disease (HD), one putative pathological mechanism reported to play a prominent role in the pathogenesis of this neurological disorder is mitochondrial dysfunction. We examined mitochondria in preferentially vulnerable striatal calbindin-positive neurons in moderate-to-severe grade HD patients, using antisera against mitochondrial markers of COX2, SOD2 and cytochrome c. Combined calbindin and mitochondrial marker immunofluorescence showed a significant and progressive grade-dependent reduction in the number of mitochondria in spiny striatal neurons, with marked alteration in size. Consistent with mitochondrial loss, there was a reduction in COX2 protein levels using western analysis that corresponded with disease severity. In addition, both mitochondrial transcription factor A, a regulator of mtDNA, and peroxisome proliferator-activated receptor-co-activator gamma-1 alpha, a key transcriptional regulator of energy metabolism and mitochondrial biogenesis, were also significantly reduced with increasing disease severity. Abnormalities in mitochondrial dynamics were observed, showing a significant increase in the fission protein Drp1 and a reduction in the expression of the fusion protein mitofusin 1. Lastly, mitochondrial PCR array profiling in HD caudate nucleus specimens showed increased mRNA expression of proteins involved in mitochondrial localization, membrane translocation and polarization and transport that paralleled mitochondrial derangement. These findings reveal that there are both mitochondrial loss and altered mitochondrial morphogenesis with increased mitochondrial fission and reduced fusion in HD. These findings provide further evidence that mitochondrial dysfunction plays a critical role in the pathogenesis of HD.
Figures
Figure 1.
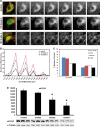
3D deconvolutional digital imaging using combined immunofluorescence of calbindin antisera identifying medium-sized spiny caudate neurons and COX2 antisera marking mitochondria from moderate Grade 2 HD and very severe Grade 4 HD patients, compared with an age-matched control subject (A). The first panel from each row represents a color image through the stacked images. Green denotes calbindin reactivity and red denotes Mt-COX2. The successive black and white panels within each row represent serial step sections through the identified neuron showing the density of mitochondrial immunostaining. Compared with the age-matched normal control, there was a significant loss of mitochondria in moderate Grade 2 HD that becomes markedly apparent in severe Grade 4 HD sections. (B) The graph represents quantitative mitochondrial binning according to size that resulted in four peaks observed in control and HD specimens. There was a progressive loss of mitochondria in HD patients. (C) The graph represents the percentile difference in mitochondrial size, as related to Grade 2 and Grade 4 HD. Consistent with the findings of COX2-positive mitochondrial loss, western analysis of brain lysates of the medial caudate nucleus in Grades 2, 3 and 4 from HD patients and age-matched control subjects showed a grade-dependent loss of COX2-immunoreactivity (D). Representative western gels of COX2 and alpha tubulin activities are present under each bar in the graph. An asterisk represents significance.
Figure 2.
3D deconvolutional digital imaging of combined immunofluorescence of calbindin antisera identifying medium-sized spiny caudate neurons and SOD2 antisera marking mitochondria from moderate Grade 2 HD and very severe Grade 4 HD patients, compared with an age-matched control subject. Consistent with the combined calbindin/COX2 results, there was a significant loss of mitochondria in moderate Grade 2 HD, with a greater loss of mitochondria in the severe Grade 4 HD sections.
Figure 3.
Cytochrome c mitochondrial immunoactivity in HD caudate nucleus. 3D deconvolutional digital imaging of combined immunofluorescence of calbindin antisera identifying medium-sized spiny caudate neurons and cytochrome c antisera marking mitochondria from moderate Grade 2 HD and very severe Grade 4 HD patients, compared with an age-matched control subject. The loss of mitochondria confirmed the findings observed in the COX2 and SOD2 studies.
Figure 4.
Western analysis of the caudate nucleus in Grades 2, 3 and 4 from HD patients showing significant grade-dependent reductions in both PGC-1α (A) and TFAM (B) in brain lysates from HD patients. Representative western gels of PGC-1α, TFAM and alpha tubulin activities are present under each bar in the graph. An asterisk represents significance.
Figure 5.
COX2-positive mitochondrial density in large cholinergic neurons within the HD neostriatum, compared with age-matched control caudate nucleus. (A and B) The images represent black and white 3D stacked images of a large striatal neuron in control caudate nucleus (A) and Grade 4 HD caudate nucleus (B). Mt-COX2-positive immunoreactive mitochondria are identified by white spheroid profiles. Asterisk shows a medium-sized striatal neuron for comparison. The bar in B represents 20 µm.
Figure 6.
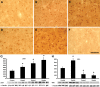
Histopathology and western analysis of Drp1 and Mfn1 activities. Immunohistochemistry of Drp1activity in the medial caudate nucleus showed a progressive increase in protein expression in Grade 2 (B) and Grade 4 (C) HD specimens, compared with a normal control patient (A). Greater immunoreactivity is observed in both neurons and in the neuropil. Mfn1 immunoreactivity was markedly increased in Grade 2 (E) HD caudate nucleus, with a significant reduction in Grade 4 (F) specimens, compared with a normal control (D). Western analysis findings paralleled the immunohistology observations characterizing both proteins in brain tissue lysate from Grades 2, 3 and 4 HD frozen specimens (G, Drp1 and H, Mfn1). The bar in F represents 100 µm. An asterisk denotes significance.
Figure 7.
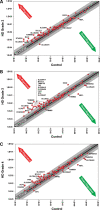
Scatter plots of gene changes from Grade 2 (A), Grade 3 (B) and Grade 4 (C) HD patients. The center black-line represents the cipher, and upregulation and downregulation are noted by the red and green arrows, respectively. One- to 2-fold (dark gray zone) and 2- to 4-fold (light gray zone) mRNA changes are represented on both sides of each figure. Downregulated mRNA changes ≥2-fold included stratifin (SFN), apoptosis-inducing factor mitochondrion-associated 2 (AFIM2), neurofilament light polypeptide (NEFL), translocase of inner mitochondrial membrane 50 homolog, translocase of outer mitochondrial membrane 70 homolog A (TOMM70A), translocase of outer mitochondrial membrane 34 (TOMM34), heat shock protein 90 kDa alpha (cytosolic) class A member (HSP90AA1) and three solute mitochondrial carrier family 25 genes (SLC25A31, adenine nucleotide translocator; SLC25A27; and SLC25A23, phosphate carrier). Those upregulated mRNA changes ≥4-fold are identified as tumor protein p53 (TP53), superoxide dismutase 2 (SOD2), stratifin (SFN), BCL2-like 1 (BCL2L1), BAK1, translocase of inner mitochondrial membrane 44 homolog (TIMM44), translocase of inner mitochondrial membrane 17 homolog B (TIMM17B), tafazzin (TAZ), fracture callus 1 homolog (FXC1), translocase of outer mitochondrial membrane 40 homolog (TOMM40), uncoupling protein 3 (mitochondrial, proton carrier) (UCP3), StAR-related lipid transfer (START) domain containing 3 (STARD3) and seven solute mitochondrial carrier family 25 genes (SLC25A37; SLC25A25, phosphate carrier; SLC25A20, carnitine/acylcarnitine translocase; SLC25A2, ornithine transporter; SLC25A17, peroxisomal membrane protein, 34 kDa; SLC25A13, citrin; and SLC25A1, citrate transporter member 1).
Similar articles
- S-nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in Huntington's disease.
Haun F, Nakamura T, Shiu AD, Cho DH, Tsunemi T, Holland EA, La Spada AR, Lipton SA. Haun F, et al. Antioxid Redox Signal. 2013 Oct 10;19(11):1173-84. doi: 10.1089/ars.2012.4928. Epub 2013 Jun 20. Antioxid Redox Signal. 2013. PMID: 23641925 Free PMC article. - Mutant huntingtin's interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington's disease.
Shirendeb UP, Calkins MJ, Manczak M, Anekonda V, Dufour B, McBride JL, Mao P, Reddy PH. Shirendeb UP, et al. Hum Mol Genet. 2012 Jan 15;21(2):406-20. doi: 10.1093/hmg/ddr475. Epub 2011 Oct 13. Hum Mol Genet. 2012. PMID: 21997870 Free PMC article. - Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington's disease: implications for selective neuronal damage.
Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, Reddy PH. Shirendeb U, et al. Hum Mol Genet. 2011 Apr 1;20(7):1438-55. doi: 10.1093/hmg/ddr024. Epub 2011 Jan 21. Hum Mol Genet. 2011. PMID: 21257639 Free PMC article. - Increased mitochondrial fission and neuronal dysfunction in Huntington's disease: implications for molecular inhibitors of excessive mitochondrial fission.
Reddy PH. Reddy PH. Drug Discov Today. 2014 Jul;19(7):951-5. doi: 10.1016/j.drudis.2014.03.020. Epub 2014 Mar 28. Drug Discov Today. 2014. PMID: 24681059 Free PMC article. Review. - Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington's disease.
Reddy PH, Shirendeb UP. Reddy PH, et al. Biochim Biophys Acta. 2012 Feb;1822(2):101-10. doi: 10.1016/j.bbadis.2011.10.016. Epub 2011 Nov 4. Biochim Biophys Acta. 2012. PMID: 22080977 Free PMC article. Review.
Cited by
- Signaling Pathways Concerning Mitochondrial Dysfunction: Implications in Neurodegeneration and Possible Molecular Targets.
Sharma Y, Gupta JK, Babu MA, Singh S, Sindhu RK. Sharma Y, et al. J Mol Neurosci. 2024 Oct 28;74(4):101. doi: 10.1007/s12031-024-02269-5. J Mol Neurosci. 2024. PMID: 39466510 Review. - The role of the gut microbiota in neurodegenerative diseases targeting metabolism.
Fu Y, Gu Z, Cao H, Zuo C, Huang Y, Song Y, Jiang Y, Wang F. Fu Y, et al. Front Neurosci. 2024 Sep 26;18:1432659. doi: 10.3389/fnins.2024.1432659. eCollection 2024. Front Neurosci. 2024. PMID: 39391755 Free PMC article. Review. - Neurodegenerative disorders, metabolic icebergs, and mitohormesis.
Phillips MCL, Picard M. Phillips MCL, et al. Transl Neurodegener. 2024 Sep 6;13(1):46. doi: 10.1186/s40035-024-00435-8. Transl Neurodegener. 2024. PMID: 39242576 Free PMC article. Review. - Mutant huntingtin impairs neurodevelopment in human brain organoids through CHCHD2-mediated neurometabolic failure.
Lisowski P, Lickfett S, Rybak-Wolf A, Menacho C, Le S, Pentimalli TM, Notopoulou S, Dykstra W, Oehler D, López-Calcerrada S, Mlody B, Otto M, Wu H, Richter Y, Roth P, Anand R, Kulka LAM, Meierhofer D, Glazar P, Legnini I, Telugu NS, Hahn T, Neuendorf N, Miller DC, Böddrich A, Polzin A, Mayatepek E, Diecke S, Olzscha H, Kirstein J, Ugalde C, Petrakis S, Cambridge S, Rajewsky N, Kühn R, Wanker EE, Priller J, Metzger JJ, Prigione A. Lisowski P, et al. Nat Commun. 2024 Aug 22;15(1):7027. doi: 10.1038/s41467-024-51216-w. Nat Commun. 2024. PMID: 39174523 Free PMC article. - Influence of dietary patterns in the pathophysiology of Huntington's Disease: A literature review.
Ansari U, Nadora D, Alam M, Wen J, Asad S, Lui F. Ansari U, et al. AIMS Neurosci. 2024 Apr 12;11(2):63-75. doi: 10.3934/Neuroscience.2024005. eCollection 2024. AIMS Neurosci. 2024. PMID: 38988882 Free PMC article. Review.
References
- Ferrante R.J., Beal M.F., Kowall N.W., Richardson E.P., Jr, Martin J.B. Sparing of acetylcholinesterase-containing striatal neurons in Huntington's disease. Brain Res. 1987;411:162–166. doi:10.1016/0006-8993(87)90694-9. - DOI - PubMed
- Hersch S.M., Rosas H.R., Ferrante R.J. Neuropathology and pathophysiology of Huntington's disease. In: Watts R.L., Koller W.C., editors. Movement Disorders: Neurologic Principles and Practice. New York, NY: McGraw-Hill; 2004. pp. 503–523.
- Stack E.C., Ferrante R.J. Huntington's disease: progress and potential in the field. Expert Opin. Investig. Drugs. 2007;16:1933–1953. doi:10.1517/13543784.16.12.1933. - DOI - PubMed
- Benard G., Bellance N., James D., Parrone P., Fernandez H., Letellier T., Rossignol R. Mitochondrial bioenergetics and structural network organization. J. Cell Sci. 2007;120:838–848. doi:10.1242/jcs.03381. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50-AG08702/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- P01-AG07232/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- P01 AG007232/AG/NIA NIH HHS/United States
- NS058793/NS/NINDS NIH HHS/United States
- R37-AG15437/AG/NIA NIH HHS/United States
- P30AG13846/AG/NIA NIH HHS/United States
- NS045806/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous