The HCMV membrane glycoprotein US10 selectively targets HLA-G for degradation - PubMed (original) (raw)
The HCMV membrane glycoprotein US10 selectively targets HLA-G for degradation
Boyoun Park et al. J Exp Med. 2010.
Abstract
Human cytomegalovirus (HCMV) encodes an endoplasmic reticulum (ER)-resident transmembrane glycoprotein, US10, expressed early in the replicative cycle of HCMV as part of the same cluster that encodes the known immunoevasins US2, US3, US6, and US11. We show that US10 down-regulates cell surface expression of HLA-G, but not that of classical class I MHC molecules. The unique and short cytoplasmic tail of HLA-G (RKKSSD) is essential in its role as a US10 substrate, and a tri-leucine motif in the cytoplasmic tail of US10 is responsible for down-regulation of HLA-G. Both the kinetics of HLA-G degradation and the mechanisms responsible appear to be distinct from those used by the US2 and US11 pathways, suggesting the existence of a third route of protein dislocation from the ER. We show that US10-mediated degradation of HLA-G interferes with HLA-G-mediated NK cell inhibition. Given the role of HLA-G in protecting the fetus from attack by the maternal immune system and in directing the differentiation of human dendritic cells to promote the evolution of regulatory T cells, HCMV likely targets the HLA-G-dependent axis of immune recognition no less efficiently than it interferes with classical class I MHC-restricted antigen presentation.
Figures
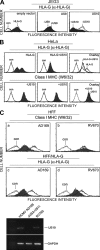
Figure 1.
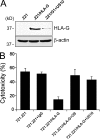
US10 down-regulates HLA-G molecules, but not classical class I MHC products, by degradation. (A) Cell surface expression of HLA-G in HCMV US9 and US10-expressing JEG3 cells. JEG3 was transiently transfected with empty vector, US9, or US10. After 48 h, cell surface expression of HLA-G was monitored by cytofluorimetry using mAb MEM-G/9. (B) US10 down-regulates HLA-G products. Both US10 and HLA-G or US10 alone were transiently transfected into HeLa cells. Surface expression of HLA-G or classical class I MHC was measured by cytofluorimetry using MEM-G/9 or W6/32. (C) Human foreskin fibroblasts (HFF) stably expressing HLA-G were infected with wild-type HCMV AD169 (a and c, shaded area), a HCMV mutant ΔUS2-US11 (a–d, solid black line), or RV670 (b and d, shaded area). Surface levels of either classical class I MHC or HLA-G by cytofluorimetry using W6/32 or MEM-G/9. US10 gene product is verified by RT-PCR in a HCMV AD169 or mutant virus-infected cells. The data shown are representative of two independent experiments with similar results.
Figure 2.
HLA-G dislocation and degradation in the presence of US10. (A) US10 degrades HLA-G. Lysates from JEG3 cells expressing HA-tagging US3, US9, or US10 were analyzed by immunoblotting with the indicated antibodies. (B) US10-expressing JEG3 cells were metabolically labeled for 20 min and chased for 6 h. Assembled HLA-G molecules were immunoprecipitated with MEM-G/9 and treated with endo H where indicated. Immunoprecipitation from NP-40 lysates of JEG3 cells (C) and HeLa cells (D) pulse-labeled for 20 min and chased for the indicated time points in the absence (lanes 1–6) or presence (lanes 7–9) of 50 µM ZL3VS were performed with MEM-G/1 antibodies. A representative result of two independent experiments is shown.
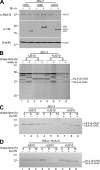
Figure 3.
Characterization of the cytoplasmic determinants of US10 responsible for the degradation of HLA-G. (A) Schematic representation of the chimeras of US10 and HLA-G. TM, transmembrane; CT, cytoplasmic tail; filled, HLA-G; open, HLA-A2. (B) Stability and maturation of US10 and its deletion mutants. HeLa cells were transfected with US10, US10ΔCT or US10ΔTM+CT. Cells were labeled for 15 min and chased for the indicated times. Immunoprecipitated proteins were digested (+) or not digested (−) with endo H. (C) HLA-G surface expression in JEG3 and HeLa cells expressing US10 chimeras. HeLa and JEG3 cells were transiently transfected with the individual cDNAs encoding the US10 deletion mutants. After 48 h, cell surface expression of HLA-G was analyzed by cytofluorimetry using MEM-G/9. Dashed line, no US10; solid line, US10; bolded line, transfectants. (D and E) Stability of HLA-G in cells expressing wild-type US10, mutants, or HA-TEV epitope–tagged constructs of US10. (F and G) The tri-leucine motif of the US10 cytoplasmic tail is critical for degradation of HLA-G. Expression of HLA-G and the indicated point mutants of US10 were analyzed by cytofluorimetry and immunoblotting using MEM-G/9 and MEM-G/1 antibodies. Data are from three independent experiments.
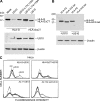
Figure 4.
US10 uniquely targets the short cytoplasmic tail of HLA-G. (A and C) HeLa cells expressed HLA-G, HLA-GΔCT, or HLA-A2.1 were transiently transfected with the indicated constructs and observed for protein expression or cell surface expression of HLA-G by immunoblotting or cytofluorimetry with the indicated antibodies (A: α-HLA-G, α-HA, or α-β-actin; C: α-HLA-G or BB7.2). (B) HeLa cells expressing US10 were transiently transfected with wild-type HLA-A2 or HLA-A/G tail and examined for protein expression of HLA-A2 by immunoblotting with HLA-A2 antibody. Dashed line, control Ab; solid line: transfectants alone; bolded line: transfectants+US10. Data are representative of similar experiments performed at least two times.
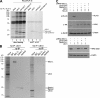
Figure 5.
US10 selectively targets HLA-G molecules for degradation by a mechanism distinct from that used by HCMV US11. (A) The luminal domain of US10 is required for the interaction of HLA-G. Cells transfected with indicated cDNA were labeled with [35S] methionine and cysteine for 1 h. The anti-HLA-G immunoprecipitate was analyzed directly (lanes 1–4) or reimmunoprecipitated after dissociation of the initial immunoprecipitate using anti-HA antibodies (lanes 5–8). (B) US10 does not obviously associate with the dislocation components recruited by US11: Sel1L and Derlin1, 2. Immunoprecipitations from digitonin lysates were performed with anti-HA antibodies. The anti-HA immunoprecipitates were either analyzed directly or reimmunoprecipitated using the indicated antibodies. (C) Expression of a Derlin1/2 dominant-negative construct or shRNA Sel1L does not inhibit US10-mediated HLA-G degradation. Data are representative of two experiments.
Figure 6.
US10 interferes with HLA-G-mediated Natural Killer cell cytotoxic activity. (A) Expression level of HLA-G by US10 in 721.221 cells was analyzed by immunoblotting with MEM-G/1 antibodies. (B) Target cells were stained with DiOC18 (3) for 30 min and then pNK cells were coincubated with different target cells at a 10:1 ratio (Effect: Target) for 5 h. After incubation time, cells were stained with propidium iodide for dead cells for 10 min and analyzed by cytofluorimetry. Data are representative of three experiments.
Similar articles
- Multimodal HLA-I genotype regulation by human cytomegalovirus US10 and resulting surface patterning.
Gerke C, Bauersfeld L, Schirmeister I, Mireisz CN, Oberhardt V, Mery L, Wu D, Jürges CS, Spaapen RM, Mussolino C, Le-Trilling VTK, Trilling M, Dölken L, Paster W, Erhard F, Hofmann M, Schlosser A, Hengel H, Momburg F, Halenius A. Gerke C, et al. Elife. 2024 Jun 20;13:e85560. doi: 10.7554/eLife.85560. Elife. 2024. PMID: 38900146 Free PMC article. - Human cytomegalovirus-encoded US2 differentially affects surface expression of MHC class I locus products and targets membrane-bound, but not soluble HLA-G1 for degradation.
Barel MT, Ressing M, Pizzato N, van Leeuwen D, Le Bouteiller P, Lenfant F, Wiertz EJ. Barel MT, et al. J Immunol. 2003 Dec 15;171(12):6757-65. doi: 10.4049/jimmunol.171.12.6757. J Immunol. 2003. PMID: 14662880 - Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: a novel mechanism for evading major histocompatibility complex class II antigen presentation.
Hegde NR, Tomazin RA, Wisner TW, Dunn C, Boname JM, Lewinsohn DM, Johnson DC. Hegde NR, et al. J Virol. 2002 Nov;76(21):10929-41. doi: 10.1128/jvi.76.21.10929-10941.2002. J Virol. 2002. PMID: 12368336 Free PMC article. - Inhibition of the MHC class II antigen presentation pathway by human cytomegalovirus.
Johnson DC, Hegde NR. Johnson DC, et al. Curr Top Microbiol Immunol. 2002;269:101-15. doi: 10.1007/978-3-642-59421-2_7. Curr Top Microbiol Immunol. 2002. PMID: 12224504 Review. - Identifying the ERAD ubiquitin E3 ligases for viral and cellular targeting of MHC class I.
van den Boomen DJ, Lehner PJ. van den Boomen DJ, et al. Mol Immunol. 2015 Dec;68(2 Pt A):106-11. doi: 10.1016/j.molimm.2015.07.005. Epub 2015 Jul 22. Mol Immunol. 2015. PMID: 26210183 Free PMC article. Review.
Cited by
- The many faces of human leukocyte antigen-G: relevance to the fate of pregnancy.
Dahl M, Djurisic S, Hviid TV. Dahl M, et al. J Immunol Res. 2014;2014:591489. doi: 10.1155/2014/591489. Epub 2014 Mar 4. J Immunol Res. 2014. PMID: 24741608 Free PMC article. Review. - Multiple E2 ubiquitin-conjugating enzymes regulate human cytomegalovirus US2-mediated immunoreceptor downregulation.
van de Weijer ML, Schuren ABC, van den Boomen DJH, Mulder A, Claas FHJ, Lehner PJ, Lebbink RJ, Wiertz EJHJ. van de Weijer ML, et al. J Cell Sci. 2017 Sep 1;130(17):2883-2892. doi: 10.1242/jcs.206839. Epub 2017 Jul 25. J Cell Sci. 2017. PMID: 28743740 Free PMC article. - NK Cell Memory to Cytomegalovirus: Implications for Vaccine Development.
Forrest C, Gomes A, Reeves M, Male V. Forrest C, et al. Vaccines (Basel). 2020 Jul 20;8(3):394. doi: 10.3390/vaccines8030394. Vaccines (Basel). 2020. PMID: 32698362 Free PMC article. Review. - Gestational Viral Infections: Role of Host Immune System.
Beltrami S, Rizzo S, Schiuma G, Speltri G, Di Luca D, Rizzo R, Bortolotti D. Beltrami S, et al. Microorganisms. 2023 Jun 22;11(7):1637. doi: 10.3390/microorganisms11071637. Microorganisms. 2023. PMID: 37512810 Free PMC article. Review. - Insights into the Transcriptome of Human Cytomegalovirus: A Comprehensive Review.
Zeng J, Cao D, Yang S, Jaijyan DK, Liu X, Wu S, Cruz-Cosme R, Tang Q, Zhu H. Zeng J, et al. Viruses. 2023 Aug 8;15(8):1703. doi: 10.3390/v15081703. Viruses. 2023. PMID: 37632045 Free PMC article. Review.
References
- Barel M.T., Ressing M., Pizzato N., van Leeuwen D., Le Bouteiller P., Lenfant F., Wiertz E.J. 2003. Human cytomegalovirus-encoded US2 differentially affects surface expression of MHC class I locus products and targets membrane-bound, but not soluble HLA-G1 for degradation. J. Immunol. 171:6757–6765 - PubMed
- Ben-Arieh S.V., Zimerman B., Smorodinsky N.I., Yaacubovicz M., Schechter C., Bacik I., Gibbs J., Bennink J.R., Yewdell J.W., Coligan J.E., et al. 2001. Human cytomegalovirus protein US2 interferes with the expression of human HFE, a nonclassical class I major histocompatibility complex molecule that regulates iron homeostasis. J. Virol. 75:10557–10562 10.1128/JVI.75.21.10557-10562.2001 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials