Plant polyphenols as dietary antioxidants in human health and disease - PubMed (original) (raw)
Review
Plant polyphenols as dietary antioxidants in human health and disease
Kanti Bhooshan Pandey et al. Oxid Med Cell Longev. 2009 Nov-Dec.
Free PMC article
Abstract
Polyphenols are secondary metabolites of plants and are generally involved in defense against ultraviolet radiation or aggression by pathogens. In the last decade, there has been much interest in the potential health benefits of dietary plant polyphenols as antioxidant. Epidemiological studies and associated meta-analyses strongly suggest that long term consumption of diets rich in plant polyphenols offer protection against development of cancers, cardiovascular diseases, diabetes, osteoporosis and neurodegenerative diseases. Here we present knowledge about the biological effects of plant polyphenols in the context of relevance to human health.
Figures
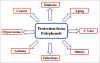
Figure 1
Pleiotropic health beneficial effects of dietary plant polyphenols: Polyphenols are naturally occurring compounds found largely in the fruits, vegetables, cereals and beverages. These molecules are secondary metabolites of plants and are generally involved in defense against ultraviolet radiation or aggression by pathogens and may also contribute to the bitterness, astringency of the food. Researchers have explored that these molecules are very good antioxidants and may neutralize the destructive reactivity of undesired reactive oxygen/nitrogen species produced as byproduct during metabolic processes in the body. Epidemiological studies have revealed that polyphenols provide a significant protection against development of several chronic diseases such as cardiovascular diseases (CVDs), cancer, diabetes, infections, aging, asthma etc.
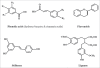
Figure 2
Chemical structures of the different classes of polyphenols. Polyphenols are classified on the basis of the number of phenol rings that they contain and of the structural elements that bind these rings to one another. They are broadly dived in four classes; Phenolic acids, flavonoids, stilbenes and lignans. Phenolic acids are further divided into hydroxyl benzoic and hydroxyl cinnamic acids. Phenolic acids account for about a third of the polyphenolic compounds in our diet and are found in all plant material, but are particularly abundant in acidic-tasting fruits. Caffeic acid, gallic acid, ferulic acid are some common phenolic acids. Flavonoids are most abundant polyphenols in human diet and share a common basic structure consist of two aromatic rings, which are bound together by three carbon atoms that form an oxygenated heterocycle. Biogenetically, one ring usually arises from a molecule of resorcinol, and other ring is derived from the shikimate pathway. Stilbenes contain two phenyl moieties connected by a twocarbon methylene bridge. Most stilbenes in plants act as antifungal phytoalexins, compounds that are synthesized only in response to infection or injury. The most extensively studied stilbene is resveratrol. Lignans are diphenolic compounds that contain a 2,3-dibenzylbutane structure that is formed by the dimerization of two cinnamic acid residues.
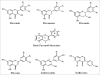
Figure 3
Chemical structures of sub-classes of flavonoids. Based on the variation in the type of heterocycle involved, flavonoids are divided into six major subclasses: flavonols, flavanones, flavanols, flavones, anthocyanins and isoflavones. Individual differences within each group arise from the variation in number and arrangement of the hydroxyl groups and their extent of alkylation and/or glycosylation. Flavonols (such as quercetin and kaempferol), have a 3-hydroxy pyran-4-one group on the C ring. Flavanones (such as naringenin and taxifolin), have an unsaturated carbon-carbon bond in the C ring. Flavanols (such as the catechins), lack both a 3-hydroxyl group and the 4-one structure in the C ring. Flavones (such as luteolin), lack a hydroxyl group in the 3-position on the C ring. Anthocyanins (such as cyanidin), are characterized by the presence of an oxonium ion on the C ring and are highly coloured as a consequence and in isoflavones (such as genistein), the B ring is attached to the C ring in the 3-position, rather than the 2-position as is the case with the other flavonoids.
Similar articles
- Important Flavonoids and Their Role as a Therapeutic Agent.
Ullah A, Munir S, Badshah SL, Khan N, Ghani L, Poulson BG, Emwas AH, Jaremko M. Ullah A, et al. Molecules. 2020 Nov 11;25(22):5243. doi: 10.3390/molecules25225243. Molecules. 2020. PMID: 33187049 Free PMC article. Review. - Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications.
Taheri Y, Suleria HAR, Martins N, Sytar O, Beyatli A, Yeskaliyeva B, Seitimova G, Salehi B, Semwal P, Painuli S, Kumar A, Azzini E, Martorell M, Setzer WN, Maroyi A, Sharifi-Rad J. Taheri Y, et al. BMC Complement Med Ther. 2020 Aug 1;20(1):241. doi: 10.1186/s12906-020-03033-z. BMC Complement Med Ther. 2020. PMID: 32738903 Free PMC article. Review. - Morin: A Promising Natural Drug.
Caselli A, Cirri P, Santi A, Paoli P. Caselli A, et al. Curr Med Chem. 2016;23(8):774-91. doi: 10.2174/0929867323666160106150821. Curr Med Chem. 2016. PMID: 26018232 Review. - Antiproliferative effects of honey and of its polyphenols: a review.
Jaganathan SK, Mandal M. Jaganathan SK, et al. J Biomed Biotechnol. 2009;2009:830616. doi: 10.1155/2009/830616. Epub 2009 Jul 19. J Biomed Biotechnol. 2009. PMID: 19636435 Free PMC article. Review.
Cited by
- Association between Dietary Antioxidants and Atherosclerotic Cardiovascular Disease in South Korea: Insights from a Comprehensive Cross-Sectional Analysis.
Kim JH, Lee ME, Hwang SM, Lee JJ, Kwon YS. Kim JH, et al. J Clin Med. 2024 Oct 11;13(20):6068. doi: 10.3390/jcm13206068. J Clin Med. 2024. PMID: 39458017 Free PMC article. - Attenuation of dermal wounds through topical application of ointment containing phenol enriched fraction of Caesalpinia mimosoides Lam.
Bhat P, Upadhya V, Hegde GR, Hegde HV, Roy S. Bhat P, et al. Front Pharmacol. 2022 Oct 13;13:1025848. doi: 10.3389/fphar.2022.1025848. eCollection 2022. Front Pharmacol. 2022. PMID: 36313327 Free PMC article. - Peanut skin extract ameliorates the symptoms of type 2 diabetes mellitus in mice by alleviating inflammation and maintaining gut microbiota homeostasis.
Xiang L, Wu Q, Osada H, Yoshida M, Pan W, Qi J. Xiang L, et al. Aging (Albany NY). 2020 Jul 22;12(14):13991-14018. doi: 10.18632/aging.103521. Epub 2020 Jul 22. Aging (Albany NY). 2020. PMID: 32699185 Free PMC article. - Vegan Diet Health Benefits in Metabolic Syndrome.
Marrone G, Guerriero C, Palazzetti D, Lido P, Marolla A, Di Daniele F, Noce A. Marrone G, et al. Nutrients. 2021 Mar 2;13(3):817. doi: 10.3390/nu13030817. Nutrients. 2021. PMID: 33801269 Free PMC article. Review. - Rosa canina Extracts Have Antiproliferative and Antioxidant Effects on Caco-2 Human Colon Cancer.
Jiménez S, Gascón S, Luquin A, Laguna M, Ancin-Azpilicueta C, Rodríguez-Yoldi MJ. Jiménez S, et al. PLoS One. 2016 Jul 28;11(7):e0159136. doi: 10.1371/journal.pone.0159136. eCollection 2016. PLoS One. 2016. PMID: 27467555 Free PMC article.
References
- Scalbert A, Manach C, Morand C, Remesy C. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. - PubMed
- Spencer JP, Abd El Mohsen MM, Minihane AM, Mathers JC. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr. 2008;99:12–22. - PubMed
- Beckman CH. Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000;57:101–110.
- Graf BA, Milbury PE, Blumberg JB. Flavonols, flavonones, flavanones and human health: Epidemological evidence. J Med Food. 2005;8:281–290. - PubMed
- Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81:317–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical