The emerging role of the thioredoxin system in angiogenesis - PubMed (original) (raw)
Review
The emerging role of the thioredoxin system in angiogenesis
Louise L Dunn et al. Arterioscler Thromb Vasc Biol. 2010 Nov.
Abstract
Although there have been a multitude of studies, the mechanisms of angiogenesis remain incompletely understood. Increasing evidence suggests that cellular redox homeostasis is an important regulator of angiogenesis. The thioredoxin (TRX) system functions as an endogenous antioxidant that can exert influence over endothelial cell function via modulation of cellular redox status. It has become apparent that the cytosolic TRX1 isoform participates in both canonical and novel angiogenic signaling pathways and may represent an avenue for therapeutic exploitation. Recent studies have further identified a role for the mitochondrial isoform TRX2 in ischemia-induced angiogenesis. TRX-interacting protein (TXNIP) is the endogenous inhibitor of TRX redox activity that has been implicated in growth factor-mediated angiogenesis. As TXNIP is strongly induced by glucose, this molecule could be of consequence to disordered angiogenesis manifest in diabetes mellitus. This review will focus on data implicating the TRX system in endothelial cell homeostasis and angiogenesis.
Figures
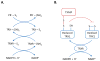
Figure 1. The Thioredoxin System
(A) Thioredoxin (TRX) in the oxidized (TRX-S2) or reduced (TRX-(SH)2) state. In the reduced state, TRX directly reduces disulfides in oxidized substrate proteins (PR--S2). The resultant oxidation of TRX in this process is reversible and maintained by thioredoxin reductase (TRXR) and the electron donor NADPH. (B) Thioredoxin interacting protein (TXNIP) can form a mixed disulfide with reduced thioredoxin-1 (TRX1), inhibiting the ability of TRX1 to reduce disulfides of other protein substrates and/or undergo reversible oxidation.
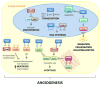
Figure 2. The Thioredoxin System and Angiogenesis
Thioredoxin-1 (TRX1) and its endogenous inhibitor Thioredoxin Interacting Protein (TXNIP) are involved in multiple signaling pathways and cellular processes that confluence in mediation of angiogenesis. The TRX1/TXNIP system can modulate cell growth and proliferation by transcriptional mechanisms such as redox factor-1 (REF1) and NF-kB, JAB1/p27kip1 translocation, DNA synthesis via ribonucleotide reductase and energy metabolism/glycolysis through reductive inhibition of PTEN. Akt signaling facilitates cell survival. Apoptosis is modulated through the interaction of either cytosolic TRX1 or mitochondrial thioredoxin-2 (TRX2) with apoptosis signaling kinase-1 (ASK1) and competitive inhibition by TXNIP. In endothelial cells TRX1 prevents von Hippel-Lindau (pVHL) mediated degradation of the transcription factor hypoxia inducible factor-1α (HIF1α) leading to induction of vascular endothelial growth factor (VEGF) expression. The VEGF signaling cascade results in the activation of endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) release that facilitates key angiogenic events.
Similar articles
- Thioredoxin-interacting protein expression is required for VEGF-mediated angiogenic signal in endothelial cells.
Abdelsaid MA, Matragoon S, El-Remessy AB. Abdelsaid MA, et al. Antioxid Redox Signal. 2013 Dec 20;19(18):2199-212. doi: 10.1089/ars.2012.4761. Epub 2013 Jul 12. Antioxid Redox Signal. 2013. PMID: 23718729 Free PMC article. - Thioredoxin1 Inactivation Mediates the Impairment of Ischemia-Induced Angiogenesis and Further Injury in Diabetic Myocardium.
Hou R, Shen M, Wang R, Liu H, Gao C, Xu J, Tao L, Yin Z, Yin T. Hou R, et al. J Vasc Res. 2020;57(2):76-85. doi: 10.1159/000505455. Epub 2020 Jan 22. J Vasc Res. 2020. PMID: 31968349 - The thioredoxin antioxidant system.
Lu J, Holmgren A. Lu J, et al. Free Radic Biol Med. 2014 Jan;66:75-87. doi: 10.1016/j.freeradbiomed.2013.07.036. Epub 2013 Jul 27. Free Radic Biol Med. 2014. PMID: 23899494 Review. - A critical role for thioredoxin-interacting protein in diabetes-related impairment of angiogenesis.
Dunn LL, Simpson PJ, Prosser HC, Lecce L, Yuen GS, Buckle A, Sieveking DP, Vanags LZ, Lim PR, Chow RW, Lam YT, Clayton Z, Bao S, Davies MJ, Stadler N, Celermajer DS, Stocker R, Bursill CA, Cooke JP, Ng MK. Dunn LL, et al. Diabetes. 2014 Feb;63(2):675-87. doi: 10.2337/db13-0417. Epub 2013 Nov 5. Diabetes. 2014. PMID: 24198286 Free PMC article. - Thioredoxin/Txnip: redoxisome, as a redox switch for the pathogenesis of diseases.
Yoshihara E, Masaki S, Matsuo Y, Chen Z, Tian H, Yodoi J. Yoshihara E, et al. Front Immunol. 2014 Jan 9;4:514. doi: 10.3389/fimmu.2013.00514. Front Immunol. 2014. PMID: 24409188 Free PMC article. Review.
Cited by
- Thioredoxin-interacting protein expression is required for VEGF-mediated angiogenic signal in endothelial cells.
Abdelsaid MA, Matragoon S, El-Remessy AB. Abdelsaid MA, et al. Antioxid Redox Signal. 2013 Dec 20;19(18):2199-212. doi: 10.1089/ars.2012.4761. Epub 2013 Jul 12. Antioxid Redox Signal. 2013. PMID: 23718729 Free PMC article. - Cytotoxic and radiosensitising effects of a novel thioredoxin reductase inhibitor in breast cancer.
Abdullah NA, Inman M, Moody CJ, Storr SJ, Martin SG. Abdullah NA, et al. Invest New Drugs. 2021 Oct;39(5):1232-1241. doi: 10.1007/s10637-021-01106-5. Epub 2021 Mar 25. Invest New Drugs. 2021. PMID: 33768386 Free PMC article. - Preimplantation embryo metabolism and culture systems: experience from domestic animals and clinical implications.
Absalón-Medina VA, Butler WR, Gilbert RO. Absalón-Medina VA, et al. J Assist Reprod Genet. 2014 Apr;31(4):393-409. doi: 10.1007/s10815-014-0179-2. Epub 2014 Feb 28. J Assist Reprod Genet. 2014. PMID: 24682781 Free PMC article. Review. - Hypoxia inhibits expression and function of mitochondrial thioredoxin 2 to promote pulmonary hypertension.
Adesina SE, Wade BE, Bijli KM, Kang BY, Williams CR, Ma J, Go YM, Hart CM, Sutliff RL. Adesina SE, et al. Am J Physiol Lung Cell Mol Physiol. 2017 May 1;312(5):L599-L608. doi: 10.1152/ajplung.00258.2016. Epub 2017 Jan 27. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28130258 Free PMC article. - The Importance of NADPH Oxidases and Redox Signaling in Angiogenesis.
Prieto-Bermejo R, Hernández-Hernández A. Prieto-Bermejo R, et al. Antioxidants (Basel). 2017 May 13;6(2):32. doi: 10.3390/antiox6020032. Antioxidants (Basel). 2017. PMID: 28505091 Free PMC article. Review.
References
- Banai S, Shweiki D, Pinson A, Chandra M, Lazarovici G, Keshet E. Upregulation of vascular endothelial growth factor expression induced by myocardial ischaemia: implications for coronary angiogenesis. Cardiovasc Res. 1994;28:1176–1179. - PubMed
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. - PubMed
- Huang SS, Zheng RL. Biphasic regulation of angiogenesis by reactive oxygen species. Pharmazie. 2006;61:223–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC2 HL103400/HL/NHLBI NIH HHS/United States
- U01 HL100397/HL/NHLBI NIH HHS/United States
- RC2HL103400/HL/NHLBI NIH HHS/United States
- K12HL08776/HL/NHLBI NIH HHS/United States
- U01HL100397/HL/NHLBI NIH HHS/United States
- K12 HL087746/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials