Distinct functions of maternal and somatic Pat1 protein paralogs - PubMed (original) (raw)
Distinct functions of maternal and somatic Pat1 protein paralogs
Aline Marnef et al. RNA. 2010 Nov.
Abstract
We previously identified Xenopus Pat1a (P100) as a member of the maternal CPEB RNP complex, whose components resemble those of P-(rocessing) bodies, and which is implicated in translational control in Xenopus oocytes. Database searches have identified Pat1a proteins in other vertebrates, as well as paralogous Pat1b proteins. Here we characterize Pat1 proteins, which have no readily discernable sequence features, in Xenopus oocytes, eggs, and early embryos and in human tissue culture cells. xPat1a and 1b have essentially mutually exclusive expression patterns in oogenesis and embryogenesis. xPat1a is degraded during meiotic maturation, via PEST-like regions, while xPat1b mRNA is translationally activated at GVBD by cytoplasmic polyadenylation. Pat1 proteins bind RNA in vitro, via a central domain, with a preference for G-rich sequences, including the NRAS 5' UTR G-quadruplex-forming sequence. When tethered to reporter mRNA, both Pat proteins repress translation in oocytes. Indeed, both epitope-tagged proteins interact with the same components of the CPEB RNP complex, including CPEB, Xp54, eIF4E1b, Rap55B, and ePAB. However, examining endogenous protein interactions, we find that in oocytes only xPat1a is a bona fide component of the CPEB RNP, and that xPat1b resides in a separate large complex. In tissue culture cells, hPat1b localizes to P-bodies, while mPat1a-GFP is either found weakly in P-bodies or disperses P-bodies in a dominant-negative fashion. Altogether we conclude that Pat1a and Pat1b proteins have distinct functions, mediated in separate complexes. Pat1a is a translational repressor in oocytes in a CPEB-containing complex, and Pat1b is a component of P-bodies in somatic cells.
Figures
FIGURE 1.
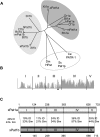
Identification of Pat1a and Pat1b proteins. (A) Unrooted phylogenetic tree of Pat1 family proteins was assembled using ClustalW (
). The abbreviations and accession numbers are as follows: Sc: S. cerevisiae (NP_010002), Sp: Schizosaccharomyces pombe (NP_595976), Ce: Caenorhabditis elegans (NP_496514), Dm: Drosophila melanogaster (NP_650592), Bt: Bos taurus Pat1a (XM_868851), B. taurus Pat1b (XP_877785), Cf: Canis familiaris Pat1a (XP_851448), C. familiaris Pat1b (XP_877785), Hs: Homo sapiens Pat1a (NP_001138584), H. sapiens Pat1b (NP_689929), Mm, Mus musculus Pat1a (AAI45647), M. musculus Pat1b (AAH58941), Rn: Rattus norvengicus Pat1a (EDL80016), R. norvengicus Pat1b (NP_001101990), Dr: Danio renio Pat1a (XP_683261), D. renio Pat1b (NP_001076497), Xl: Xenopus laevis Pat1a (NP_001085311), X. laevis Pat1b (AAH98995), Xt: Xenopus tropicalis Pat1a (NP_001135679), X. tropicalis Pat1b (Xt7.1-TTbA027g22.3.5, which is derived from assembled scaffolds of the Gurdon Institute X. tropicalis full-length database). Light gray circle denotes vertebrate Pat1a proteins, dark gray circle vertebrate Pat1b, and white circle invertebrates and yeast. (B) Similarity plot of the alignment of eight pairs of vertebrate Pat1 proteins using the Align X module of Vector NTI (Invitrogen) showing the five delineated regions of xPat1 proteins, where the height of the peak indicates degree of similarity. The asterisk in region III indicates the conserved helix called “helix x.” (C) Schematic representation of X. laevis Pat1a and Pat1b protein regions (not to scale). The identity (ID) and similarity (sim) scores between xPat1a and xPat1b are also indicated (calculated with the Ebi, EMBOSS pairwise alignment tool).
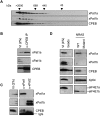
FIGURE 2.
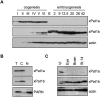
xPat1a and xPat1b expression profile in oogenesis and embryogenesis. (A) The expression levels of xPat1a and xPat1b were analyzed by Western blot using stage I–VI oocyte, egg, and different embryonic stages lysates (the stages of embryogenesis correspond to two-cell embryo; stage 9, midblastula; stage 12.5, gastrula; stage 20, neural fold closure; stage 26, tail-bud stage; stage 42, tadpole-like stage). Two cell equivalents were loaded. Actin was used as a loading control. (B) Both xPat1a and xPat1b are cytoplasmic proteins. Two cell equivalents of total (T), cytoplasmic (C), and nuclear (N) fractions from stage VI oocytes were analyzed by Western blot. PARN was used as nuclear control (Copeland and Wormington 2001). (C) xPat1b is expressed in eyes and brains of stage 42 embryos (tadpole-like stage). Actin was used as a loading control. One stage VI oocyte, 10 eyes, five brains of stage 42 embryos, and one stage 14 embryo were analyzed by Western blot.
FIGURE 3.
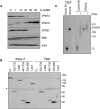
xPat1a is degraded whereas xPat1b is newly synthesized upon meiotic maturation. (A) Meiotic maturation time course scored as percentage of GVBD. Groups of stage VI oocytes treated with progesterone were sampled at different times, corresponding to maturation status scored in percentage of GVBD. The oocytes were analyzed by Western blot and probed with the indicated antibodies. (B) The N-terminal 1–331 amino acids mediate xPat1a degradation. mRNAs encoding for different MS2-tagged portions of the protein as indicated were injected in stage VI oocytes, subsequently matured into eggs by the addition of progesterone. Lysates were prepared from oocytes and eggs and analyzed by Western blotting with an MS2 antibody. Note that region 1–331 runs aberrantly in the SDS-PAGE gel. (*)Nonspecific band, serving as a loading control. (C) xPat1b mRNA is polyadenylated upon meiotic maturation. The 32[P]-labeled 3′ proximal 180 nt of xPat1b 3′ UTR and the 3′ proximal 65 nt of cyclin B1 3′ UTR were injected in stage VI oocytes (−) and subsequently matured into eggs (+) by the addition of progesterone. Input lanes contain uninjected RNA. 32[P]-labeled ØX174-HindIII fragments served as size markers.
FIGURE 4.
xPat1 proteins bind RNA in vitro via a central region. (A) xPat1 proteins bind RNA homopolymers in vitro. Autoradiograph of in vitro–translated and 35[S]-Met–labeled xPat1a, xPat1b, hPat1b, and CPEB (control) bound to poly(A), poly(U), poly(C), poly(G), and Sepharose beads (B, as a control for unspecific binding). (B) xPat1a and xPat1b poly(G) binding is competed by wild-type, but not mutant, NRAS G-quadruplex–forming RNAs (Kumari et al. 2007). Approximately 5, 10, 20, and 40 molar equivalents of NRAS relative to poly(G) homopolymer were used as competitors, and the levels of Pat1 proteins resistant to competition were quantitated by densitometry. (C) xPat1 proteins bind RNA via region III (RIII). Graph of the percentage binding of different regions of xPat1 proteins and CPEB to the RNA homopolymers. (D) Helix x in region III is required for RNA binding. Graph of the percentage of binding in full length (FL), constructs lacking RIII (ΔRIII), or helix x (Δhelix x) bound to poly(U) and poly(G) homopolymers. Three independent experiments were performed and standard error bars are shown (C,D).
FIGURE 5.
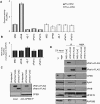
MS2-xPat1a and -xPat1b repress translation when tethered and interact with CPEB RNP components. (A) xPat1 proteins repress translation when tethered. mRNAs encoding MS2-tagged xPat1a, -xPat1b, and control mRNAs encoding MS2, ePAB, and 4E-T were injected into stage VI oocytes. Six hours after the first injection, firefly luciferase mRNA reporter (Fluc) (m7GpppG-capped but nonpolyadenylated) containing 3′ UTR MS2 hairpins, was coinjected with Renilla luciferase (Rluc), used as an internal control (dark gray). Firely luciferase reporter mRNA lacking the MS2 hairpins was used as a control (light gray). The ratios of the luciferase activities were normalized to the one observed with MS2 alone. Three independent experiments were performed and standard deviation bars are shown. (B) qPCR showing equal levels of the injected RNAs. The qPCR was first normalized to GAPDH levels, and the relative ratio between luciferase and Renilla reporter mRNAs was subsequently normalized to MS2. Three independent experiments were performed and standard error bars are shown. (C) CPEB antibody immunoprecipitates tagged xPat1a and xPat1b. mRNAs encoding the MS2-Flag–tagged xPat1a, xPat1b were injected in stage VI oocytes. The immunoprecipitated proteins were analyzed by Western blotting with the indicated antibodies. (D) mRNAs encoding the MS2-Flag–tagged xPat1a, xPat1b were injected in stage VI oocytes, some of which were matured with progesterone into eggs. Immunoprecipitation was carried out using Flag antibody, and the Western blot was analyzed with the indicated antibodies. *Phosphorylated form of Flag-xPat1b. One stage VI oocyte was loaded as an input (2%).
FIGURE 6.
Endogenous xPat1a is a component of the CPEB complex, not endogenous xPat1b. (A) xPat1a and xPat1b coelute with CPEB in a large complex of ∼3 M Da in a Superose 6 HR10/30 gel filtration column loaded with stage V/VI oocyte lysate. Column fractions were analyzed by Western blotting, with the indicated antibodies. (B) CPEB antibody coimmunoprecipitates endogenous xPat1a but not xPat1b in oocytes. (C) xPat1a antibody coimmunoprecipitates CPEB, whereas anti-xPat1b antibody does not. (D) NRAS RNA G-quadruplex interacts with the CPEB repression complex. Wild-type biotinylated NRAS RNA G-quadruplex interacts preferentially with components of the CPEB complex as compared with a biotinylated mutant sequence.
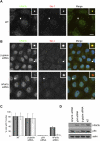
FIGURE 7.
Endogenous hPat1b localizes to P-bodies and is not absolutely required for their formation. (A) Confocal imaging of HeLa cells stained with Pat1b antibody, with Ge-1 as a P-body marker. The white arrows point to the zoomed P-body (white box). Scale bar, 10 μm. (B) Depletion of hPat1b does not prevent P-body formation. hPat1b, p54, and β-globin siRNAs were transfected into HeLa cells, which were fixed and stained with hPat1b and Ge-1 antibodies 48 h post-transfection and visualized by fluorescence microscopy. The white arrows point to the zoomed P-body (white boxes). Scale bar, 10 μm. (C) Percentage of cells with P-bodies. The graph represents the percentage of cells with P-bodies as seen with either hPat1b (light gray) or Ge-1 (dark gray) antibodies, in nontransfected (NT) cells, or in cells transfected with control β-globin, p54, or hPat1b siRNA. Three independent experiments were performed and standard deviation bars are shown. (D) Western blot of the depletion effect of the different siRNAs used (30 μg protein loaded), with indicated antibodies.
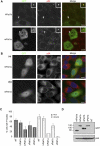
FIGURE 8.
Pat1b-GFP localizes to P-bodies while Pat1a-GFP localizes weakly to P-bodies in some cells but exerts a dominant-negative effect on P-bodies in others. (A) Confocal imaging of HeLa cells transfected with hPat1b-GFP or mPat1a-GFP. Cells were fixed 24 h post-transfection and stained with p54 antibodies as a P-body marker. White arrow points to the zoomed P-body (white box). Scale bar, 10 μm. (B) Fluorescent imaging of HeLa cells transfected with mPat1a-GFP showing two phenotypes: cells with P-bodies (a) or lacking P-bodies (b). Cells were fixed 24 h post-transfection and stained with p54 as a P-body marker. (C) Graph of the percentage of cells containing P-bodies (as seen with p54) when transfected with different GFP constructs in HeLa and HEK293 cells. Three independent experiments were performed and standard deviation bars are shown. (NT) Nontransfected cells. (D) The GFP constructs are expressed at similar levels in HeLa cells. Sixty micrograms protein was loaded on a SDS-PAGE gel and analyzed by Western blotting with the indicated antibodies.
Similar articles
- CPEB interacts with an ovary-specific eIF4E and 4E-T in early Xenopus oocytes.
Minshall N, Reiter MH, Weil D, Standart N. Minshall N, et al. J Biol Chem. 2007 Dec 28;282(52):37389-401. doi: 10.1074/jbc.M704629200. Epub 2007 Oct 17. J Biol Chem. 2007. PMID: 17942399 - CPEB and miR-15/16 Co-Regulate Translation of Cyclin E1 mRNA during Xenopus Oocyte Maturation.
Wilczynska A, Git A, Argasinska J, Belloc E, Standart N. Wilczynska A, et al. PLoS One. 2016 Feb 1;11(2):e0146792. doi: 10.1371/journal.pone.0146792. eCollection 2016. PLoS One. 2016. PMID: 26829217 Free PMC article. - The active form of Xp54 RNA helicase in translational repression is an RNA-mediated oligomer.
Minshall N, Standart N. Minshall N, et al. Nucleic Acids Res. 2004 Feb 24;32(4):1325-34. doi: 10.1093/nar/gkh303. Print 2004. Nucleic Acids Res. 2004. PMID: 14982957 Free PMC article. - Translational control by cytoplasmic polyadenylation in Xenopus oocytes.
Radford HE, Meijer HA, de Moor CH. Radford HE, et al. Biochim Biophys Acta. 2008 Apr;1779(4):217-29. doi: 10.1016/j.bbagrm.2008.02.002. Epub 2008 Feb 14. Biochim Biophys Acta. 2008. PMID: 18316045 Free PMC article. Review. - Translational control by CPEB: a means to the end.
Mendez R, Richter JD. Mendez R, et al. Nat Rev Mol Cell Biol. 2001 Jul;2(7):521-9. doi: 10.1038/35080081. Nat Rev Mol Cell Biol. 2001. PMID: 11433366 Review.
Cited by
- The conserved P body component HPat/Pat1 negatively regulates synaptic terminal growth at the larval Drosophila neuromuscular junction.
Pradhan SJ, Nesler KR, Rosen SF, Kato Y, Nakamura A, Ramaswami M, Barbee SA. Pradhan SJ, et al. J Cell Sci. 2012 Dec 15;125(Pt 24):6105-16. doi: 10.1242/jcs.113043. Epub 2012 Oct 24. J Cell Sci. 2012. PMID: 23097047 Free PMC article. - RNA-related nuclear functions of human Pat1b, the P-body mRNA decay factor.
Marnef A, Weil D, Standart N. Marnef A, et al. Mol Biol Cell. 2012 Jan;23(1):213-24. doi: 10.1091/mbc.E11-05-0415. Epub 2011 Nov 16. Mol Biol Cell. 2012. PMID: 22090346 Free PMC article. - Visualization and selective chemical targeting of RNA G-quadruplex structures in the cytoplasm of human cells.
Biffi G, Di Antonio M, Tannahill D, Balasubramanian S. Biffi G, et al. Nat Chem. 2014 Jan;6(1):75-80. doi: 10.1038/nchem.1805. Epub 2013 Nov 24. Nat Chem. 2014. PMID: 24345950 Free PMC article. - Fungal virulence and development is regulated by alternative pre-mRNA 3'end processing in Magnaporthe oryzae.
Franceschetti M, Bueno E, Wilson RA, Tucker SL, Gómez-Mena C, Calder G, Sesma A. Franceschetti M, et al. PLoS Pathog. 2011 Dec;7(12):e1002441. doi: 10.1371/journal.ppat.1002441. Epub 2011 Dec 15. PLoS Pathog. 2011. PMID: 22194688 Free PMC article. - Translational repression of cyclin D3 by a stable G-quadruplex in its 5' UTR: implications for cell cycle regulation.
Weng HY, Huang HL, Zhao PP, Zhou H, Qu LH. Weng HY, et al. RNA Biol. 2012 Aug;9(8):1099-109. doi: 10.4161/rna.21210. Epub 2012 Aug 1. RNA Biol. 2012. PMID: 22858673 Free PMC article.
References
- Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W 2006. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125: 1111–1124 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous