Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation - PubMed (original) (raw)
Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation
S-W Hong et al. Allergy. 2011 Mar.
Free PMC article
Abstract
Background: Recently, we found that Staphylococcus aureus produces extracellular vesicles (EV) that contain pathogenic proteins. Although S. aureus infection has been linked with atopic dermatitis (AD), the identities of the causative agents from S. aureus are controversial. We evaluated whether S. aureus-derived EV are causally related to the pathogenesis of AD.
Methods: Extracellular vesicles were isolated by the ultracentrifugation of S. aureus culture media. The EV were applied three times per week to tape-stripped mouse skin. Inflammation and immune dysfunction were evaluated 48 h after the final application in hairless mice. Extracellular vesicles-specific IgE levels were measured by ELISA in AD patients and healthy subjects.
Results: The in vitro application of S. aureus EV increased the production of pro-inflammatory mediators (IL-6, thymic stromal lymphopoietin, macrophage inflammatory protein-1α, and eotaxin) by dermal fibroblasts. The in vivo application of S. aureus EV after tape stripping caused epidermal thickening with infiltration of the dermis by mast cells and eosinophils in mice. These changes were associated with the enhanced cutaneous production of IL-4, IL-5, IFN-γ, and IL-17. Interestingly, the serum levels of S. aureus EV-specific IgE were significantly increased in AD patients relative to healthy subjects.
Conclusion: These results indicate that S. aureus EV induce AD-like inflammation in the skin and that S. aureus-derived EV are a novel diagnostic and therapeutic target for the control of AD.
© 2010 John Wiley & Sons A/S.
Figures
Figure 1
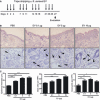
Staphylococcus aureus extracellular vesicles (EV) enhance the in vitro secretion of immune and pro-inflammatory mediators by mouse dermal fibroblasts. (A) Scanning electron microscopic images that S. aureus secrete EV. Arrowheads identify S. aureus EV. (B) Levels of pro-inflammatory mediators IL-6, thymic stromal lymphopoietin, macrophage inflammatory protein-1α and eotaxin in supernatants of dermal fibroblasts after stimulation with EV and >100- and < 100-kD soluble fractions of bacterial culture media. (C) Western blotting to detect staphylococcal enterotoxin B (SEB) in EV and soluble (Sup) fractions of S. aureus culture media. (D) Levels of pro-inflammatory mediators from supernatants of dermal fibroblasts after stimulation with EV or SEB. Assays were performed in duplicate. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 2
Application of Staphylococcus aureus extracellular vesicles (EV) to tape-stripped mouse skin induces atopic dermatitis-like inflammation. (A) Study protocol: application of different doses of S. aureus EV to tape-stripped mouse skin for 4 weeks (n = 5 per treatment group). (B) Skin histology [H&E staining; magnification, ×200 (upper panel) and ×400 (lower panel)]. Arrowheads identify eosinophils. (C) Histological analysis of epidermal thickness and the numbers of eosinophils and mast cells infiltrating the dermis. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 3
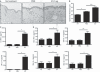
Three-week exposure of tape-stripped mouse skin to Staphylococcus aureus extracellular vesicles (EV) induces a mixed Th1-/Th17-/Th2-type inflammatory response in the skin, and the generation of Th1 and Th17 cells in skin-draining lymph nodes (LNs). Evaluation (n = 5 per treatment group) was performed 48 h after the final application of S. aureus EV (5 μg) (performed three times a week for 3 weeks) to tape-stripped skin. *P < 0.05; **P < 0.01; ***P < 0.001. (A) Skin histology [H&E staining; magnification, ×200]. (B) Histological analysis of epidermal thickness. (C) Levels of IFN-γ and IL-17 in supernatants from S. aureus EV-treated cells from skin-draining LNs. (D) Levels of IFN-γ, IL-17, IL-4, and IL-5 in skin tissue homogenates.
Figure 4
Long-term exposure of tape-stripped mouse skin to Staphylococcus aureus extracellular vesicles (EV) enhances the production of IgE. Evaluation (n = 5 per treatment group) was performed 48 h after the final application of S. aureus EV (5 μg) (performed three times a week for 8 weeks) to tape-stripped skin. *P < 0.05; **P < 0.01; ***P < 0.001. (A) Skin histology [H&E staining; magnification, ×200 (upper panel) and ×400 (lower panel)]. Arrowheads identify eosinophils. (B) Histological analysis of epidermal thickness and numbers of eosinophils and mast cells infiltrating the dermis. (C) Levels of IL-17 in supernatants from S. aureus EV-treated cells from skin-draining lymph nodes. (D) Serum levels of total IgG1 and total IgE.
Figure 5
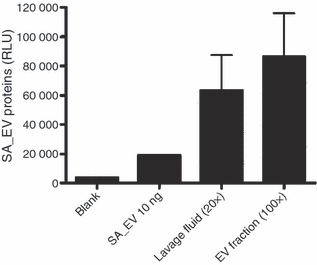
_Staphylococcus aureus_-derived extracellular vesicles (EV) are present on the skin of atopic dermatitis (AD) patients. This figure showed ELISA assay to detect S. aureus EV-specific proteins using anti-S. aureus EV polyclonal antibodies; lavage fluids and EV fraction of lavage fluids obtained from two AD patients have S. aureus EV-specific proteins (SA_EV, _S. aureus_-derived EV).
Figure 6
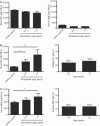
Staphylococcus aureus extracellular vesicles (EV)-specific IgE is elevated in atopic dermatitis (AD) patients than in age-matched healthy subjects. (A) Levels of S. aureus EV-specific (left panel) and SEB-specific (right panel) IgG1 in serum from AD patients and healthy subjects. (B) Levels of S. aureus EV-specific IgE in serum from AD patients and healthy subjects (left panel), and positive rate of elevated S. aureus EV-specific IgE in AD patients (right panel). (C) Levels of SEB-specific IgE in serum from AD patients and healthy subjects (left panel), and positive rate of elevated SEB-specific IgE in AD patients (right panel). Serum samples from AD patients (n = 30 in patients aged 6–9 years, and n = 30 in patients aged 9–16 years) and healthy subjects aged 6–16 years (n = 20); EV, _S. aureus_-derived EV; SEB, staphylococcal enterotoxin B. *P < 0.05; **P < 0.01.
Similar articles
- An important role of α-hemolysin in extracellular vesicles on the development of atopic dermatitis induced by Staphylococcus aureus.
Hong SW, Choi EB, Min TK, Kim JH, Kim MH, Jeon SG, Lee BJ, Gho YS, Jee YK, Pyun BY, Kim YK. Hong SW, et al. PLoS One. 2014 Jul 3;9(7):e100499. doi: 10.1371/journal.pone.0100499. eCollection 2014. PLoS One. 2014. PMID: 24992681 Free PMC article. - Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression.
Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, Hata TR, Gallo RL. Nakatsuji T, et al. J Invest Dermatol. 2016 Nov;136(11):2192-2200. doi: 10.1016/j.jid.2016.05.127. Epub 2016 Jul 2. J Invest Dermatol. 2016. PMID: 27381887 Free PMC article. - NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation.
Jiao D, Wong CK, Qiu HN, Dong J, Cai Z, Chu M, Hon KL, Tsang MS, Lam CW. Jiao D, et al. Cell Mol Immunol. 2016 Jul;13(4):535-50. doi: 10.1038/cmi.2015.77. Epub 2015 Sep 21. Cell Mol Immunol. 2016. PMID: 26388234 Free PMC article. - The Role of Immune Defects and Colonization of Staphylococcus aureus in the Pathogenesis of Atopic Dermatitis.
Nowicka D, Grywalska E. Nowicka D, et al. Anal Cell Pathol (Amst). 2018 May 2;2018:1956403. doi: 10.1155/2018/1956403. eCollection 2018. Anal Cell Pathol (Amst). 2018. PMID: 29854575 Free PMC article. Review. - Role of bacterial pathogens in atopic dermatitis.
Lin YT, Wang CT, Chiang BL. Lin YT, et al. Clin Rev Allergy Immunol. 2007 Dec;33(3):167-77. doi: 10.1007/s12016-007-0044-5. Clin Rev Allergy Immunol. 2007. PMID: 18163223 Review.
Cited by
- Staphylococcus aureus extracellular vesicles carry biologically active β-lactamase.
Lee J, Lee EY, Kim SH, Kim DK, Park KS, Kim KP, Kim YK, Roh TY, Gho YS. Lee J, et al. Antimicrob Agents Chemother. 2013 Jun;57(6):2589-95. doi: 10.1128/AAC.00522-12. Epub 2013 Mar 25. Antimicrob Agents Chemother. 2013. PMID: 23529736 Free PMC article. - Atopic dermatitis: molecular, cellular, and clinical aspects.
Salimian J, Salehi Z, Ahmadi A, Emamvirdizadeh A, Davoudi SM, Karimi M, Korani M, Azimzadeh Jamalkandi S. Salimian J, et al. Mol Biol Rep. 2022 Apr;49(4):3333-3348. doi: 10.1007/s11033-021-07081-7. Epub 2022 Jan 6. Mol Biol Rep. 2022. PMID: 34989960 Review. - Emerging role of extracellular vesicles in inflammatory diseases.
Buzas EI, György B, Nagy G, Falus A, Gay S. Buzas EI, et al. Nat Rev Rheumatol. 2014 Jun;10(6):356-64. doi: 10.1038/nrrheum.2014.19. Epub 2014 Feb 18. Nat Rev Rheumatol. 2014. PMID: 24535546 Review. - GPER activation protects against epithelial barrier disruption by Staphylococcus aureus α-toxin.
Triplett KD, Pokhrel S, Castleman MJ, Daly SM, Elmore BO, Joyner JA, Sharma G, Herbert G, Campen MJ, Hathaway HJ, Prossnitz ER, Hall PR. Triplett KD, et al. Sci Rep. 2019 Feb 4;9(1):1343. doi: 10.1038/s41598-018-37951-3. Sci Rep. 2019. PMID: 30718654 Free PMC article. - Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi.
Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Brown L, et al. Nat Rev Microbiol. 2015 Oct;13(10):620-30. doi: 10.1038/nrmicro3480. Epub 2015 Sep 1. Nat Rev Microbiol. 2015. PMID: 26324094 Free PMC article. Review.
References
- Fiset PO, Leung DY, Hamid Q. Immunopathology of atopic dermatitis. J Allergy Clin Immunol. 2006;118:287–290. - PubMed
- Tupker RA, De Monchy JG, Coenraads PJ, Homan A, van der Meer JB. Induction of atopic dermatitis by inhalation of house dust mite. J Allergy Clin Immunol. 1996;97:1064–1070. - PubMed
- Maintz L, Novak N. Getting more and more complex: the pathophysiology of atopic eczema. Eur J Dermatol. 2007;17:267–283. - PubMed
- Breuer K, Häussler S, Kapp A, Werfel T. Staphylococcus aureus: colonizing features and influence of an antibacterial treatment in adults with atopic dermatitis. Br J Dermatol. 2002;147:55–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources