Allele-specific copy number analysis of tumors - PubMed (original) (raw)
. 2010 Sep 28;107(39):16910-5.
doi: 10.1073/pnas.1009843107. Epub 2010 Sep 13.
Silje H Nordgard, Ole Christian Lingjærde, Hege G Russnes, Inga H Rye, Wei Sun, Victor J Weigman, Peter Marynen, Anders Zetterberg, Bjørn Naume, Charles M Perou, Anne-Lise Børresen-Dale, Vessela N Kristensen
Affiliations
- PMID: 20837533
- PMCID: PMC2947907
- DOI: 10.1073/pnas.1009843107
Allele-specific copy number analysis of tumors
Peter Van Loo et al. Proc Natl Acad Sci U S A. 2010.
Abstract
We present an allele-specific copy number analysis of the in vivo breast cancer genome. We describe a unique bioinformatics approach, ASCAT (allele-specific copy number analysis of tumors), to accurately dissect the allele-specific copy number of solid tumors, simultaneously estimating and adjusting for both tumor ploidy and nonaberrant cell admixture. This allows calculation of "ASCAT profiles" (genome-wide allele-specific copy-number profiles) from which gains, losses, copy number-neutral events, and loss of heterozygosity (LOH) can accurately be determined. In an early-stage breast carcinoma series, we observe aneuploidy (>2.7n) in 45% of the cases and an average nonaberrant cell admixture of 49%. By aggregation of ASCAT profiles across our series, we obtain genomic frequency distributions of gains and losses, as well as genome-wide views of LOH and copy number-neutral events in breast cancer. In addition, the ASCAT profiles reveal differences in aberrant tumor cell fraction, ploidy, gains, losses, LOH, and copy number-neutral events between the five previously identified molecular breast cancer subtypes. Basal-like breast carcinomas have a significantly higher frequency of LOH compared with other subtypes, and their ASCAT profiles show large-scale loss of genomic material during tumor development, followed by a whole-genome duplication, resulting in near-triploid genomes. Finally, from the ASCAT profiles, we construct a genome-wide map of allelic skewness in breast cancer, indicating loci where one allele is preferentially lost, whereas the other allele is preferentially gained. We hypothesize that these alternative alleles have a different influence on breast carcinoma development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
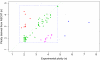
Fig. 1.
ASCAT profiles and their calculation. Two examples are given: (A) a tumor with ploidy close to 2n and (B) a tumor with ploidy close to 4n. (Left) ASCAT first determines the ploidy of the tumor cells ψ_t_ and the fraction of aberrant cells ρ. This procedure evaluates the goodness of fit for a grid of possible values for both parameters (blue, good solution; red, bad solution; detailed in Materials and Methods). On the basis of this goodness of fit, the optimal solution is selected (green cross). Using the resulting tumor ploidy and aberrant cell fraction, an ASCAT profile is calculated (Upper Right), containing the allele-specific copy number of all assayed loci [copy number on the y axis vs. the genomic location on the x axis; green, allele with lowest copy number; red, allele with highest copy number; for illustrative purposes only, both lines are slightly shifted (red, down; green, up) such that they do not overlap; only probes heterozygous in the germline are shown]. Finally, for all aberrations found, an aberration reliability score is calculated (Lower Right).
Fig. 2.
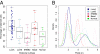
Validation of tumor ploidy predicted by ASCAT. ASCAT's ploidy estimates are plotted relative to experimentally measured ploidy. Here we define ploidy as the amount of DNA relative to a haploid genome. For 58 of the 79 assayed samples (73.4%), ASCAT's ploidy predictions correspond well with the experimentally determined ploidy (green triangles close to the diagonal). Three samples (3.8%) have an experimentally determined ploidy larger than 5n (blue triangles), outside of the ploidy range used by ASCAT (1.6n–4.8n, depicted by the blue square). Ten breast carcinomas (12.7%) have a predicted ploidy close to 2n, whereas the experimentally determined ploidy was close to 4n (pink triangles). For most of these cases, manual inspection of the copy number profiles could not reveal any indications (missed by ASCAT) that these samples are in fact close to tetraploid (
Fig. S5_A_
). Indeed, cases that are tetraploid but only show even-numbered allele-specific copy numbers would be recognized as diploid, because the SNP array data do not provide any information to distinguish such tetraploid samples from diploid samples. Alternatively, the experimental method for ploidy determination, applied to a different part of the tumor as the SNP arrays, could be measuring tumor cells in the S phase of the cell cycle, or a different subclone of the tumor. Finally, eight samples (10.1%) show clearly higher ploidy by ASCAT prediction compared with the experimentally determined ploidy (red triangles). A possible explanation for this is the presence of multiple populations of aberrant tumor cells with (slightly) different aberrations (
Fig. S5_B_
).
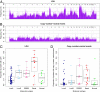
Fig. 3.
Percentage of aberrant tumor cells and ploidy across the five breast cancer subtypes. Molecular subtypes used: LumA, Luminal A (n = 45); LumB, Luminal B (n = 10); ERBB2 (n = 12); Basal, Basal-like (n = 12); Normal, Normal-like (n = 8). (A) Distribution of percentage of aberrant tumor cells across the five subtypes. The box plots show the median (thick lines) and the lower and upper quartile (boxes). The whiskers reach up to the most extreme value within 1.5 times the interquartile range from the box. Whereas Luminal A carcinomas harbored the highest levels of aberrant tumor cells (P = 6.9 × 10−6, unpaired t test with unequal variance, testing for differences between the Luminal A subtype and all other carcinomas), tumors of the ERBB2 and Normal-like subtype displayed the lowest fraction of aberrant cells (P = 3.7 × 10−4 and P = 8.4 × 10−3, respectively). (B) Distribution of ploidy across the five subtypes. The vast majority of Luminal A tumors showed a ploidy close to 2n, with a smaller fraction showing a ploidy close to 4n. Carcinomas of the Luminal B subtype were approximately equally divided among 2n and 4n tumors, with two tumors being 3n. On average, the ERBB2 subgroup displayed the highest level of ploidy but also the broadest range. The Basal-like subgroup showed cases with a ploidy 1.6n–2n and cases of 2.8n–3.2n. Normal-like tumors showed a group of cases with ploidy close to 2n and a group of cases with ploidy above 3n.
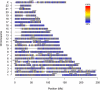
Fig. 4.
Frequency of LOH and copy number-neutral events. (A) Frequency of LOH across the genome. Probes are shown in genomic order along the x axis, from chromosome 1 to chromosome X, where different chromosomes are delimited by gray lines. (B) Frequency of copy number-neutral events across the genome. For diploid tumors, copy number-neutral events correspond to a subset of LOH (copy number-neutral LOH), but for, for example, tetraploid tumors, a copy number-neutral event can also be three copies of A and one copy of B. (C) Proportion of LOH per case (percentage of probes heterozygous in the germline that have lost this heterozygosity in the tumor), stratified by molecular breast cancer subtypes. Molecular subtypes used and box plot legends are the same as in Fig. 3. The Luminal A subtype shows a significantly lower frequency of LOH compared with the four other subtypes (P = 2.3 × 10−6, unpaired t test with unequal variance). Even more striking is the elevated level of LOH for the Basal-like subtype (P = 1.0 × 10−3). Indeed, two thirds of the Basal-like tumors show LOH at more than 40% of the loci heterozygous in the germline. (D) Proportion of copy number-neutral events per case, stratified by molecular breast cancer subtypes. The Luminal A (P = 4.7 × 10−3, unpaired t test with unequal variance, testing for differences between the Luminal A subtype and all other carcinomas) and Normal-like (P = 0.95) subtype display low levels of copy number-neutral events, the Luminal B subgroup shows intermediate levels (P = 0.99), and the Basal-like (P = 0.043) and ERBB2 subtypes (P = 0.064) show the highest frequencies of copy number-neutral events.
Fig. 5.
Genome-wide map of allelic skewness. SNPs that show no allelic skewness (no allele is preferentially gained or lost) should show approximately equal frequencies of loss and gain for both alleles. Here, the frequency of the most frequently gained/lost allele is shown. Alleles without allelic skewness should have a frequency of 50% (blue), whereas alleles that are completely skewed have a frequency of 100% (red). For each SNP, we selected the cases from our series that are germline heterozygous. We count how many cases show gains (of A vs. of B), losses (of A vs. of B), and copy number-neutral events (with gain of A and loss of B vs. with gain of B and loss of A). We combined the counts for gain of A, loss of B, and copy number-neutral events with gain of A and loss of B, and the counts for gain of B, loss of A, and copy number-neutral events with gain of B and loss of A, and display the frequency of the most frequently skewed allele. Only probes with a total of at least 10 gains, losses, and copy number-neutral events are shown. Gene symbols shown contain at least one SNP with a most frequently gained/lost allele frequency of 95% or more.
Similar articles
- Landscape of somatic allelic imbalances and copy number alterations in HER2-amplified breast cancer.
Staaf J, Jönsson G, Ringnér M, Baldetorp B, Borg A. Staaf J, et al. Breast Cancer Res. 2011;13(6):R129. doi: 10.1186/bcr3075. Epub 2011 Dec 14. Breast Cancer Res. 2011. PMID: 22169037 Free PMC article. - Analyzing cancer samples with SNP arrays.
Van Loo P, Nilsen G, Nordgard SH, Vollan HK, Børresen-Dale AL, Kristensen VN, Lingjærde OC. Van Loo P, et al. Methods Mol Biol. 2012;802:57-72. doi: 10.1007/978-1-61779-400-1_4. Methods Mol Biol. 2012. PMID: 22130873 - Landscape of somatic allelic imbalances and copy number alterations in human lung carcinoma.
Staaf J, Isaksson S, Karlsson A, Jönsson M, Johansson L, Jönsson P, Botling J, Micke P, Baldetorp B, Planck M. Staaf J, et al. Int J Cancer. 2013 May 1;132(9):2020-31. doi: 10.1002/ijc.27879. Epub 2012 Oct 20. Int J Cancer. 2013. PMID: 23023297 - [Analysis of genomic copy number alterations of malignant lymphomas and its application for diagnosis].
Tagawa H. Tagawa H. Gan To Kagaku Ryoho. 2007 Jul;34(7):975-82. Gan To Kagaku Ryoho. 2007. PMID: 17637530 Review. Japanese. - Analysis of genomic abnormalities in tumors: a review of available methods for Illumina two-color SNP genotyping and evaluation of performance.
Oros KK, Arcand SL, Bayani J, Squire JA, Mes-Masson AM, Tonin PN, Greenwood CM. Oros KK, et al. Cancer Genet. 2013 Apr;206(4):103-15. doi: 10.1016/j.cancergen.2013.03.001. Epub 2013 Apr 25. Cancer Genet. 2013. PMID: 23623180 Review.
Cited by
- Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics.
Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavaré S. Sottoriva A, et al. Proc Natl Acad Sci U S A. 2013 Mar 5;110(10):4009-14. doi: 10.1073/pnas.1219747110. Epub 2013 Feb 14. Proc Natl Acad Sci U S A. 2013. PMID: 23412337 Free PMC article. - Gamma Knife Radiosurgery does not alter the copy number aberration profile in sporadic vestibular schwannoma.
Håvik AL, Bruland O, Dhayalan D, Lund-Johansen M, Knappskog PM. Håvik AL, et al. J Neurooncol. 2020 Sep;149(3):373-381. doi: 10.1007/s11060-020-03631-4. Epub 2020 Sep 27. J Neurooncol. 2020. PMID: 32980934 Free PMC article. - Multi-dimensional scaling techniques unveiled gain1q&loss13q co-occurrence in Multiple Myeloma patients with specific genomic, transcriptional and adverse clinical features.
Terragna C, Poletti A, Solli V, Martello M, Zamagni E, Pantani L, Borsi E, Vigliotta I, Mazzocchetti G, Armuzzi S, Taurisano B, Testoni N, Marzocchi G, Kanapari A, Pistis I, Tacchetti P, Mancuso K, Rocchi S, Rizzello I, Cavo M. Terragna C, et al. Nat Commun. 2024 Feb 20;15(1):1551. doi: 10.1038/s41467-024-45000-z. Nat Commun. 2024. PMID: 38378709 Free PMC article. - TAFFYS: An Integrated Tool for Comprehensive Analysis of Genomic Aberrations in Tumor Samples.
Liu Y, Li A, Feng H, Wang M. Liu Y, et al. PLoS One. 2015 Jun 25;10(6):e0129835. doi: 10.1371/journal.pone.0129835. eCollection 2015. PLoS One. 2015. PMID: 26111017 Free PMC article. - Concordance between Three Homologous Recombination Deficiency (HRD) Assays in Patients with High-Grade Epithelial Ovarian Cancer.
Fountzilas E, Papadopoulou K, Chatzikonstantinou T, Karakatsoulis G, Constantoulakis P, Tsantikidi A, Tsaousis G, Karageorgopoulou S, Koumarianou A, Mauri D, Ntavatzikos A, Saridaki Z, Petrakis G, Fostira F, Fountzilas G, Liontos M. Fountzilas E, et al. Cancers (Basel). 2023 Nov 22;15(23):5525. doi: 10.3390/cancers15235525. Cancers (Basel). 2023. PMID: 38067228 Free PMC article.
References
- Balmain A, Gray J, Ponder B. The genetics and genomics of cancer. Nat Genet. 2003;33(Suppl):238–244. - PubMed
- Mullighan CG, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases