The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase - PubMed (original) (raw)
. 2010 Nov 26;285(48):37159-69.
doi: 10.1074/jbc.M110.152942. Epub 2010 Sep 21.
Zheng-Yu Zha, Xin Zhou, Heng Zhang, Wei Huang, Di Zhao, Tingting Li, Siew Wee Chan, Chun Jye Lim, Wanjin Hong, Shimin Zhao, Yue Xiong, Qun-Ying Lei, Kun-Liang Guan
Affiliations
- PMID: 20858893
- PMCID: PMC2988322
- DOI: 10.1074/jbc.M110.152942
The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase
Chen-Ying Liu et al. J Biol Chem. 2010.
Abstract
The TAZ transcription co-activator promotes cell proliferation and epithelial-mesenchymal transition. TAZ is inhibited by the Hippo tumor suppressor pathway, which promotes TAZ cytoplasmic localization by phosphorylation. We report here that TAZ protein stability is controlled by a phosphodegron recognized by the F-box protein β-TrCP and ubiquitylated by the SCF/CRL1(β-TrCP) E3 ligase. The interaction between TAZ and β-TrCP is regulated by the Hippo pathway. Phosphorylation of a phosphodegron in TAZ by LATS primes it for further phosphorylation by CK1ε and subsequent binding by β-TrCP. Therefore, the Hippo pathway negatively regulates TAZ function by both limiting its nuclear accumulation and promoting its degradation. The phosphodegron-mediated TAZ degradation plays an important role in negatively regulating TAZ biological functions.
Figures
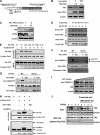
FIGURE 1.
TAZ is an unstable protein associated with β-TrCP. A, TAZ is an unstable protein. Both HeLa and MCF10A cells were treated CHX (20 μg/ml) for indicated times. Endogenous TAZ protein levels were determined. Relative TAZ levels were quantified by the ratio of TAZ to actin. B, MG132 increases TAZ protein levels in multiple cell lines. Cells were treated with either solvent DMSO or 10 μ
m
MG132. Cell lysates were analyzed by Western blot (WB). Relative TAZ levels were normalized by actin and quantified by the ratio between with and without MG132 treatment. C and D, TAZ binds to β-TrCP. HA-β-TrCP was co-transfected with FLAG-TAZ into HEK293T cells as indicated. β-TrCP and TAZ associations were examined by reciprocal co-IP as indicated. E, Cullin-1 expression decreases TAZ protein levels. TAZ was co-transfected with different cullins as indicated. The steady state level of TAZ was determined by WB. F, β-TrCP knockdown increases TAZ protein levels. Three β-TrCP RNAi oligos were individually transfected into 293 cells as indicated. β-TrCP and TAZ levels were determined by WB. Relative TAZ level were quantified by the ratio of TAZ to actin.
FIGURE 2.
Phosphorylation of Ser-311 is important for TAZ to bind with β-TrCP. A, schematic illustration of TAZ structure showing the two putative phosphodegrons. The sequences surrounding the phosphodegrons are shown. Lats phosphorylation serine residues are indicated in gray. B, ectopic expression of LATS2 decreases the steady state level of TAZ. The indicated plasmids were co-transfected into HEK293 cells. K/R denotes the kinase inactive mutant LATS2. Relative TAZ levels were quantified by the ratio of TAZ to actin. C, LATS2 knocking down increases endogenous TAZ protein level. LATS2 or unrelated control shRNA was transfected into 293 cells, and endogenous TAZ protein level was examined. LATS2 knockdown efficiency was determined by WB. Relative TAZ levels were quantified by the ratio of TAZ to actin. D, mutation of the LATS phosphorylation site S311A disrupts the interaction between TAZ and β-TrCP. β-TrCP was co-transfected with TAZ WT or different mutants. FLAG-TAZ was immunoprecipitated and the associated HA-β-TrCP was detected by HA WB. The TAZ S311A mutant showed weak interaction with β-TrCP. E, mutations of the C-terminal phosphodegron disrupt the binding between TAZ and β-TrCP. Experiments were similar to those in panel D. Different TAZ mutants used in the transfection are indicated. F, Ser-311 is phosphorylated by LATS in vitro. His-TAZ and S311A mutant were expressed in E. coli and purified. HA-LATS2 was immunoprecipitated from transfected 293T cells and used to phosphorylate the purified His-TAZ in vitro. Phosphorylation of TAZ was detected by pTAZ (Ser-311) antibody. His-TAZ was shown by Coomassie Blue staining. G, expression of LATS enhances TAZ Ser-311 phosphorylation in transfected cells. TAZ WT or S311A mutant was co-transfected with WT or kinase inactive (K/R) mutant of LATS2. Phosphorylation of the co-transfected TAZ was detected by the pTAZ(311) antibody. H, MST/LATS co-transfection increases the interaction between TAZ and β-TrCP. Indicated plasmids were co-transfected into HEK293 cells. FLAG-β-TrCP was immunoprecipitated with FLAG antibody and the co-precipitated HA-TAZ was detected by HA WB. I, TAZ protein level decreases with cell density. MCF10A cells were cultured from 30% density to confluent. TAZ protein levels were determined by WB. J, leptomycin B treatment results in a detectable increase of TAZ stability. Both Hela and Flag-TAZ expressed MCF10A cells were pretreated with or without 20 n
m
leptomycin B for 5 h, followed by treatment with CHX (20 μg/ml) for indicated times. Endogenous or Flag-TAZ protein levels were determined.
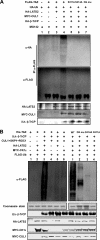
FIGURE 3.
Casein kinase I cooperates with LATS to regulate the interaction between TAZ and β-TrCP. A, IC261 treatment disrupts the interaction between TAZ and β-TrCP. The indicated plasmids were co-transfected and cells were treated with or without IC261, which is a CKIϵ/δ specific inhibitor. The interaction between TAZ and β-TrCP was analyzed by co-IP followed by WB. B, CK1ϵ promotes the interaction between TAZ and β-TrCP. The indicated plasmids were co-transfected. The association between TAZ and β-TrCP was examined by co-IP. R178C is an active mutant of CKIϵ. C, association between CK1ϵ and TAZ. CKIϵ was co-transfected with or without TAZ. co-IP was performed to examine the interaction between TAZ and CKIϵ. D, kinase inactive LATS2 mutant blocks the interaction between TAZ and β-TrCP induced by CK1ϵ. The indicated plasmids were co-transfected into HEK293 cells. Interaction between TAZ and β-TrCP was analyzed by co-IP. E, CK1ϵ activity is required for Mst2/LATS2 to stimulate the interaction between TAZ and β-TrCP. The indicated plasmids were co-transfected, and cells were treated with or without IC261 or D4476. The interaction between TAZ and β-TrCP was analyzed by co-IP. F, phosphorylation of TAZ S314 by CKIϵ requires LATS2 priming phosphorylation. His-TAZ WT or mutants were expressed and purified from E. coli. The purified TAZ was incubated with HA-LATS2 immunoprecipitated from transfected HEK293T cells, in the presence of cold ATP. HA-LATS2 was removed from the kinase reaction. The prime phosphorylated His-TAZ was then incubated with Myc-CK1ϵ immunoprecipitated from transfected HEK293T cells, in the presence of radioactive ATP. Phosphorylation of TAZ by CKIϵ was detected by incorporation of 32P. His-TAZ input was shown by Coomassie Blue staining (bottom panel).
FIGURE 4.
LATS and CK1 promote TAZ degradation via the C-terminal phosphodegron. A, Ser-311 and Ser-314 are important for TAZ degradation. MCF10A cells stably expressing TAZWT, TAZS311A, TAZS3114A, and TAZDG were chased with CHX treatment as indicted. TAZ stability was determined by WB. Relative TAZ levels were quantified by the ratio of TAZ to actin. B, Ser-311 and Ser-314 are required for TAZ destabilization by LATS and CK1ϵ. TAZ WT and different mutants were co-transfected with or without LATS and/or CK1ϵ as indicated. TAZ expression level was determined by WB. The co-transfected GFP and endogenous actin were included as controls. C, CK1 inhibitor increases endogenous TAZ protein levels. HeLa cells were treated with or without IC261 at different doses and time points. WB was performed to determine TAZ expression levels along the actin control. D, C-terminal phosphodegron is required for TAZ stabilization by CK1 inhibitors. The indicated plasmids were transfected into 293T cells. Treatments with or without the IC261 were indicated. TAZ expression level was determined by WB.
FIGURE 5.
LATS2 and CK1ϵ promote TAZ ubiquitylation by SCFβ-TrCP. A, LATS and SCFβ-TrCP E3 ligase promotes TAZ ubiquitylation depending on the C-terminal phosphodegron. FALG-TAZ was co-transfected with various plasmids as indicated. FLAG-TAZ was immunoprecipitated and ubiquitylation of the precipitated TAZ was determined by WB for the co-transfected HA-ubiquitin or FLAG-TAZ. TAZ S311A, S314A, and DG mutants were also tested for in vivo ubiquitylation. B, in vitro ubiquitylation of TAZ by SCFβ-TrCP E3 ligase requires LATS and CK1ϵ. HA-LATS2 and MYC-CK1ϵ were immunopre cipitated from transfected 293T cells. In vitro ubiquitylation assays were performed using purified His-TAZ as a substrate in the presence of various proteins as indicated. Experiments with different TAZ mutants were also performed (right panel). Ubiquitylation of TAZ was detected by FLAG antibody for FLAG-Ub. His-TAZ input was shown by Coomassie Blue staining. Other components were determined by WB as indicated.
FIGURE 6.
Phosphodegron mutants promote TAZ function. A, TAZ degradation mutants induce a stronger morphological change (upper panel) and altered actin organization in MCF10A cells (lower panel). Phase-contrast images of MCF10A cells expressing vector, TAZ and TAZS311A are shown. Cells were stained with rhodamine-conjugated phalloidin. B, TAZ degradation mutants are more potent in inducing EMT in MCF10A cells. Cell lysates from MCF10A cells expressing vector, TAZ and TAZS311A were probed for epithelial marker and mesenchymal marker as indicated. C, TAZ degradation mutants stimulate cell migration. MCF10A cells expressing vector, TAZ and TAZS311A were analyzed for migration by a wound healing assay. D, transformation of NIH 3T3 cells by TAZ degradation mutants. 5 × 103 NIH3T3 cells stably expressing vector, TAZ and TAZS311A were cultured in soft agar for 14 days before colonies were counted. Colonies were then visualized by crystal violet staining and counted.
Similar articles
- The N-terminal phosphodegron targets TAZ/WWTR1 protein for SCFβ-TrCP-dependent degradation in response to phosphatidylinositol 3-kinase inhibition.
Huang W, Lv X, Liu C, Zha Z, Zhang H, Jiang Y, Xiong Y, Lei QY, Guan KL. Huang W, et al. J Biol Chem. 2012 Jul 27;287(31):26245-53. doi: 10.1074/jbc.M112.382036. Epub 2012 Jun 12. J Biol Chem. 2012. PMID: 22692215 Free PMC article. - The nonreceptor tyrosine kinase c-Src attenuates SCF(β-TrCP) E3-ligase activity abrogating Taz proteasomal degradation.
Shanzer M, Adler J, Ricardo-Lax I, Reuven N, Shaul Y. Shanzer M, et al. Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):1678-1683. doi: 10.1073/pnas.1610223114. Epub 2017 Feb 1. Proc Natl Acad Sci U S A. 2017. PMID: 28154141 Free PMC article. - A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP).
Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. Zhao B, et al. Genes Dev. 2010 Jan 1;24(1):72-85. doi: 10.1101/gad.1843810. Genes Dev. 2010. PMID: 20048001 Free PMC article. - Hippo-Yap/Taz signaling: Complex network interactions and impact in epithelial cell behavior.
van Soldt BJ, Cardoso WV. van Soldt BJ, et al. Wiley Interdiscip Rev Dev Biol. 2020 May;9(3):e371. doi: 10.1002/wdev.371. Epub 2019 Dec 11. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 31828974 Free PMC article. Review. - Reciprocal regulation of YAP/TAZ by the Hippo pathway and the Small GTPase pathway.
Jang JW, Kim MK, Bae SC. Jang JW, et al. Small GTPases. 2020 Jul;11(4):280-288. doi: 10.1080/21541248.2018.1435986. Epub 2018 Apr 20. Small GTPases. 2020. PMID: 29457552 Free PMC article. Review.
Cited by
- Large tumor suppressor homologs 1 and 2 regulate mouse liver progenitor cell proliferation and maturation through antagonism of the coactivators YAP and TAZ.
Yi J, Lu L, Yanger K, Wang W, Sohn BH, Stanger BZ, Zhang M, Martin JF, Ajani JA, Chen J, Lee JS, Song S, Johnson RL. Yi J, et al. Hepatology. 2016 Nov;64(5):1757-1772. doi: 10.1002/hep.28768. Epub 2016 Sep 30. Hepatology. 2016. PMID: 27531557 Free PMC article. - Targeting Ferroptosis by Ubiquitin System Enzymes: A Potential Therapeutic Strategy in Cancer.
Meng Y, Sun H, Li Y, Zhao S, Su J, Zeng F, Deng G, Chen X. Meng Y, et al. Int J Biol Sci. 2022 Aug 29;18(14):5475-5488. doi: 10.7150/ijbs.73790. eCollection 2022. Int J Biol Sci. 2022. PMID: 36147464 Free PMC article. Review. - Hepatic Hippo signaling inhibits development of hepatocellular carcinoma.
Liu Y, Wang X, Yang Y. Liu Y, et al. Clin Mol Hepatol. 2020 Oct;26(4):742-750. doi: 10.3350/cmh.2020.0178. Epub 2020 Sep 28. Clin Mol Hepatol. 2020. PMID: 32981290 Free PMC article. Review. - Signal integration in TGF-β, WNT, and Hippo pathways.
Attisano L, Wrana JL. Attisano L, et al. F1000Prime Rep. 2013 Jun 3;5:17. doi: 10.12703/P5-17. Print 2013. F1000Prime Rep. 2013. PMID: 23755364 Free PMC article. - Integrin α2β1 inhibits MST1 kinase phosphorylation and activates Yes-associated protein oncogenic signaling in hepatocellular carcinoma.
Wong KF, Liu AM, Hong W, Xu Z, Luk JM. Wong KF, et al. Oncotarget. 2016 Nov 22;7(47):77683-77695. doi: 10.18632/oncotarget.12760. Oncotarget. 2016. PMID: 27765911 Free PMC article.
References
- Harvey K., Tapon N. (2007) Nat. Rev. Cancer 7, 182–191 - PubMed
- Saucedo L. J., Edgar B. A. (2007) Nat. Rev. Mol. Cell Biol. 8, 613–621 - PubMed
- Pan D. (2007) Genes Dev. 21, 886–897 - PubMed
- Udan R. S., Kango-Singh M., Nolo R., Tao C., Halder G. (2003) Nat. Cell Biol. 5, 914–920 - PubMed
- Wu S., Huang J., Dong J., Pan D. (2003) Cell 114, 445–456 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources