Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis - PubMed (original) (raw)
Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis
Tiago Tomaz et al. Plant Physiol. 2010 Nov.
Abstract
Malate dehydrogenase (MDH) catalyzes a reversible NAD(+)-dependent-dehydrogenase reaction involved in central metabolism and redox homeostasis between organelle compartments. To explore the role of mitochondrial MDH (mMDH) in Arabidopsis (Arabidopsis thaliana), knockout single and double mutants for the highly expressed mMDH1 and lower expressed mMDH2 isoforms were constructed and analyzed. A mmdh1mmdh2 mutant has no detectable mMDH activity but is viable, albeit small and slow growing. Quantitative proteome analysis of mitochondria shows changes in other mitochondrial NAD-linked dehydrogenases, indicating a reorganization of such enzymes in the mitochondrial matrix. The slow-growing mmdh1mmdh2 mutant has elevated leaf respiration rate in the dark and light, without loss of photosynthetic capacity, suggesting that mMDH normally uses NADH to reduce oxaloacetate to malate, which is then exported to the cytosol, rather than to drive mitochondrial respiration. Increased respiratory rate in leaves can account in part for the low net CO(2) assimilation and slow growth rate of mmdh1mmdh2. Loss of mMDH also affects photorespiration, as evidenced by a lower postillumination burst, alterations in CO(2) assimilation/intercellular CO(2) curves at low CO(2), and the light-dependent elevated concentration of photorespiratory metabolites. Complementation of mmdh1mmdh2 with an mMDH cDNA recovered mMDH activity, suppressed respiratory rate, ameliorated changes to photorespiration, and increased plant growth. A previously established inverse correlation between mMDH and ascorbate content in tomato (Solanum lycopersicum) has been consolidated in Arabidopsis and may potentially be linked to decreased galactonolactone dehydrogenase content in mitochondria in the mutant. Overall, a central yet complex role for mMDH emerges in the partitioning of carbon and energy in leaves, providing new directions for bioengineering of plant growth rate and a new insight into the molecular mechanisms linking respiration and photosynthesis in plants.
Figures
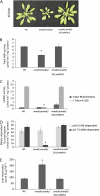
Figure 1.
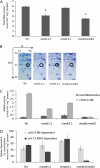
mmdh1mmdh2 knockout plants in Arabidopsis show reduced plant growth. A, RT-PCR from total RNA with primers for mMDH1 and mMDH2 compared with Actin2 control for mmdh mutants. B, Plant growth of single mmdh1, mmdh2, and mmdh1-2mmdh2 plants compared with the wild type (Wt) in long-day and short-day growth conditions at 25 and 40 d of growth, respectively.
Figure 2.
mMDH protein abundance and activity are altered in mmdh knockout plants. A, Changes in total cellular MDH activity. Bars are means ±
se
(n = 4), and asterisks indicate significant differences (P < 0.05) from the wild type (Wt). B, Loss of mMDH1 on two-dimensional gels of mitochondrial proteins from mmdh1 and mmdh1mmdh2. C, Total MDH activity in isolated mitochondria, showing activity in intact mitochondria and activity after organelle rupture with Triton X-100. Bars are means ±
se
(n = 3). D, Malate-dependent respiratory rate of isolated intact mitochondria at pH 6.5 to maximize NAD-ME activity and at pH 7.5 to maximize MDH activity. Bars are means ±
se
(n = 3).
Figure 3.
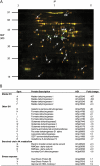
Differential in-gel analysis of mitochondria shows compensatory changes in the mitochondrial proteome in mmdh1mmdh2. A, Overlay of fluorescence images of typical two-dimensional gels showing common proteins in yellow, protein decreased in mmdh1mmdh2 in green, and protein enhanced in mmdh1mmdh2 in red. B, Proteins identified from protein spots marked in A with fold changes (n = 3; P < 0.05). AGI, Arabidopsis Genome Initiative number.
Figure 4.
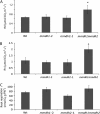
Impact of MDH loss on respiratory rate of leaves and roots. A, Respiratory CO2 production of lighted leaves in gas phase measured by the method of Laisk. Bars are means ±
sd
(n > 3), and asterisks indicate significant differences (P < 0.05) from the wild type (Wt). B, CO2 production in darkened leaves in gas phase. Bars are means ±
sd
(n > 3), and asterisks indicate significant differences (P < 0.05) from the wild type. C, Root respiration as oxygen consumption rate in liquid phase. Bars are means ±
se
(n = 3). FW, Fresh weight. Plants were grown under short-day conditions.
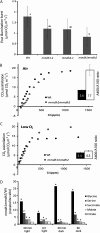
Figure 5.
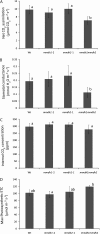
Changes in net CO2 assimilation rate and photosynthetic performance measurements in mmdh mutants. A, Net CO2 assimilation rate under growth conditions. B, Stomatal conductance to water vapor under growth conditions. C, Internal CO2 concentration under growth conditions. D, Maximum photosynthetic electron transport rate (ETC) inferred from A/Ci curves. Bars are means ±
sd
(n > 3), and letters designate groups that significantly differ (P < 0.05). Wt, Wild type. Plants were grown under short-day conditions.
Figure 6.
Changes in photorespiration in mmdh mutants. A, PIB. Bars are means ±
sd
(n = 4). B and C, A/Ci curves in air and low oxygen in the wild type (Wt) and mmdh1mmdh2. The inset graphs show A400/A100 ratios. Bars are mean ratios ±
sd
(n = 3). D, Metabolite ratios between the wild type and mmdh1mmdh2 at different times during the day and night. Fold changes with significant differences (P < 0.05; n = 5) are indicated with asterisks. Plants were grown under short-day conditions.
Figure 7.
Growth and biochemical characterization of mmdh1mmdh2 complemented with mMDH. A, Plant growth in short-day growth conditions at 40 d of age in the wild type (Wt), mmdh1mmdh2, and a complemented mmdh1mmdh2 35S:mMDH line. B, Changes in total cellular MDH activity. Bars are means ±
se
(n = 4). C, Total MDH activity in isolated mitochondria, showing activity in intact mitochondria and activity after organelle rupture with Triton X-100. Bars are means ±
se
(n = 3). D, Malate-dependent respiratory rate of isolated intact mitochondria at pH 6.5 to maximize NAD-ME activity and at pH 7.5 to maximize MDH activity. Bars are means ±
se
(n = 3). E, Changes in leaf respiration rate measured as oxygen consumption rate in the dark. Bars are means ±
se
(n = 5). FW, Fresh weight.
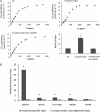
Figure 8.
Physiological and metabolite characterization of mmdh1mmdh2 complemented with mMDH. A, A/Ci curves of the wild type (Wt), mmdh1mmd2, and mmdh1mmdh2 35S:mMDH1 and A400/A100 ratios, where bars are mean ratios ±
sd
(n = 3). B, Metabolite ratios between the wild type and mmdh1mmdh2 and between the wild type and mmdh1mmdh2 35S:mMDH1 at 3 h into the day. Fold changes with significant differences (P < 0.05; n = 5) are indicated with asterisks. Plants were grown under short-day conditions.
Figure 9.
Change in GLDH protein amount and total cellular ascorbate levels in mmdh1mmdh2. A, Immunoreactivity of GLDH in different mutants on a mitochondrial protein basis. A representative gel is shown, and mean abundance of the immuonreactive band ±
se
is shown above the lanes (n = 3). The arrow indicates a truncated product of GLDH found in DIGE (Fig. 3) and also immunodetected. B, Reduced and oxidized ascorbate (AsA) content of leaf tissue. Bars are means ±
se
(n = 4), and asterisks indicate significant differences (P < 0.05) from the wild type. FW, Fresh weight; Wt, wild type. Plants were grown under short-day conditions.
Similar articles
- Loss of Mitochondrial Malate Dehydrogenase Activity Alters Seed Metabolism Impairing Seed Maturation and Post-Germination Growth in Arabidopsis.
Sew YS, Ströher E, Fenske R, Millar AH. Sew YS, et al. Plant Physiol. 2016 Jun;171(2):849-63. doi: 10.1104/pp.16.01654. Epub 2016 Apr 12. Plant Physiol. 2016. PMID: 27208265 Free PMC article. - Peroxisomal malate dehydrogenase is not essential for photorespiration in Arabidopsis but its absence causes an increase in the stoichiometry of photorespiratory CO2 release.
Cousins AB, Pracharoenwattana I, Zhou W, Smith SM, Badger MR. Cousins AB, et al. Plant Physiol. 2008 Oct;148(2):786-95. doi: 10.1104/pp.108.122622. Epub 2008 Aug 6. Plant Physiol. 2008. PMID: 18685043 Free PMC article. - Adenine nucleotide-dependent and redox-independent control of mitochondrial malate dehydrogenase activity in Arabidopsis thaliana.
Yoshida K, Hisabori T. Yoshida K, et al. Biochim Biophys Acta. 2016 Jun;1857(6):810-8. doi: 10.1016/j.bbabio.2016.03.001. Epub 2016 Mar 3. Biochim Biophys Acta. 2016. PMID: 26946085 - Malate valves: old shuttles with new perspectives.
Selinski J, Scheibe R. Selinski J, et al. Plant Biol (Stuttg). 2019 Jan;21 Suppl 1(Suppl Suppl 1):21-30. doi: 10.1111/plb.12869. Epub 2018 Jul 17. Plant Biol (Stuttg). 2019. PMID: 29933514 Free PMC article. Review. - Malate dehydrogenase in plants: evolution, structure, and a myriad of functions.
Baird LM, Berndsen CE, Monroe JD. Baird LM, et al. Essays Biochem. 2024 Oct 3;68(2):221-233. doi: 10.1042/EBC20230089. Essays Biochem. 2024. PMID: 38868915 Review.
Cited by
- QTL mapping and omics analysis to identify genes controlling kernel dehydration in maize.
Jin X, Zhang X, Wang P, Liu J, Zhang H, Wu X, Song R, Fu Z, Chen S. Jin X, et al. Theor Appl Genet. 2024 Sep 26;137(10):233. doi: 10.1007/s00122-024-04715-9. Theor Appl Genet. 2024. PMID: 39325221 - Molecular Characteristics of the Malate Dehydrogenase (MDH) Gene Family in Spirometra mansoni (Cestoda: Diphyllobothriidea).
Wang R, Hao J, Cao C, Li J, Zhang X. Wang R, et al. Int J Mol Sci. 2024 Aug 13;25(16):8802. doi: 10.3390/ijms25168802. Int J Mol Sci. 2024. PMID: 39201488 Free PMC article. - Integrating Proteomics and Metabolomics Approaches to Elucidate the Mechanism of Responses to Combined Stress in the Bell Pepper (Capsicum annuum).
Morales-Merida BE, Grimaldi-Olivas JC, Cruz-Mendívil A, Villicaña C, Valdez-Torres JB, Heredia JB, León-Chan RG, Lightbourn-Rojas LA, Monribot-Villanueva JL, Guerrero-Analco JA, Ruiz-May E, León-Félix J. Morales-Merida BE, et al. Plants (Basel). 2024 Jul 5;13(13):1861. doi: 10.3390/plants13131861. Plants (Basel). 2024. PMID: 38999705 Free PMC article. - Alternative electron pathways of photosynthesis power green algal CO2 capture.
Peltier G, Stoffel C, Findinier J, Madireddi SK, Dao O, Epting V, Morin A, Grossman A, Li-Beisson Y, Burlacot A. Peltier G, et al. Plant Cell. 2024 Oct 3;36(10):4132-4142. doi: 10.1093/plcell/koae143. Plant Cell. 2024. PMID: 38739547 Free PMC article.
References
- Alhagdow M, Mounet F, Gilbert L, Nunes-Nesi A, Garcia V, Just D, Petit J, Beauvoit B, Fernie AR, Rothan C, et al. (2007) Silencing of the mitochondrial ascorbate synthesizing enzyme l-galactono-1,4-lactone dehydrogenase affects plant and fruit development in tomato. Plant Physiol 145: 1408–1422 - PMC - PubMed
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
- Bardel J, Louwagie M, Jaquinod M, Jourdain A, Luche S, Rabilloud T, Macherel D, Garin J, Bourguignon J. (2002) A survey of the plant mitochondrial proteome in relation to development. Proteomics 2: 880–898 - PubMed
- Bartoli CG, Guiamet JJ, Kiddle G, Pastori GM, Di Cagno R, Theodoulou FL, Foyer CH. (2005) Ascorbate content of wheat leaves is not determined by maximal L-galactono-1,4-lactone dehydrogenase (GalLDH) activity under drought stress. Plant Cell Environ 28: 1073–1081
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases