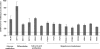Butyrate-rich colonic microenvironment is a relevant selection factor for metabolically adapted tumor cells - PubMed (original) (raw)
Butyrate-rich colonic microenvironment is a relevant selection factor for metabolically adapted tumor cells
Jacinta Serpa et al. J Biol Chem. 2010.
Abstract
The short chain fatty acid (SCFA) butyrate is a product of colonic fermentation of dietary fibers. It is the main source of energy for normal colonocytes, but cannot be metabolized by most tumor cells. Butyrate also functions as a histone deacetylase (HDAC) inhibitor to control cell proliferation and apoptosis. In consequence, butyrate and its derived drugs are used in cancer therapy. Here we show that aggressive tumor cells that retain the capacity of metabolizing butyrate are positively selected in their microenvironment. In the mouse xenograft model, butyrate-preselected human colon cancer cells gave rise to subcutaneous tumors that grew faster and were more angiogenic than those derived from untreated cells. Similarly, butyrate-preselected cells demonstrated a significant increase in rates of homing to the lung after intravenous injection. Our data showed that butyrate regulates the expression of VEGF and its receptor KDR at the transcriptional level potentially through FoxM1, resulting in the generation of a functional VEGF:KDR autocrine growth loop. Cells selected by chronic exposure to butyrate express higher levels of MMP2, MMP9, α2 and α3 integrins, and lower levels of E-cadherin, a marker for epithelial to mesenchymal transition. The orthotopic model of colon cancer showed that cells preselected by butyrate are able to colonize the animals locally and at distant organs, whereas control cells can only generate a local tumor in the cecum. Together our data shows that a butyrate-rich microenvironment may select for tumor cells that are able to metabolize butyrate, which are also phenotypically more aggressive.
Figures
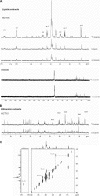
FIGURE 1.
HCT15 colon cancer cells are able to fully metabolize butyrate. A, 13C NMR spectra of the lipid fraction of HCT15 and SW480 control cells, treated with butyric acid, and sodium [U-13C]butyrate showed that in HCT15 the presence of butyrate induces a slight change in lipid profile and its carbons are incorporated in all positions of triacylglycerides; B, 13C NMR spectra of ethanolic extracts of HCT15 control cells and treated with sodium [U-13C]butyrate showed that carbons from butyrate are incorporated in carbons 4 and 5 of glutamate and carbon 5 of praline; and C, 13C-13C NMR COSY spectra of the lipid extract of HCT15 cells treated with 20% [U-13C]butyrate and 80% of butyric acid, showed only cross-peaks between two 13C indicating that butyrate is metabolized into acetate prior to incorporation.
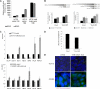
FIGURE 2.
The expression of enzymes and transporters are altered after butyrate exposure. A, Western blotting for MCAD and SCAD showed that these oxidation enzymes are increased in cells preselected by butyrate (p < 0.05); B, luciferase reporter gene assay showed that both SCAD promoter sequences −119 to −1 and −399 to −1 are significantly stimulated in cells preselected by butyric acid (p = 0.0278 for pSCAD1 and p = 0.037 for pSCAD3), the same results were observe for the MCAD promoter sequences −201 to −1 and −421 to −1 (p = 0.0213 for pMCAD1 and p = 0.0158 for pMCAD3); C, RQ-PCR showed, in HCT15, a decreased expression of mRNAs from glucose transporters GLUT1 and SGLT1, whereas MCT1 is increased in HCT15 cells preselected by butyrate; the mRNAs encoding the HEXII from glycolysis and glucose-6-phosphate dehydrogenase (G6PD) from the pentose-phosphate pathway are decreased, whereas pyruvate kinase (PKLR) from glycolysis and phosphoenolpyruvate carboxykinase (PEPCK) from gluconeogenesis are increased; in SW480, mRNA related to GLUT1, MCT1, and G6PD are increased; and E, immunoreactivity for cytochrome oxidase 2 showed mitochondria aggregation in butyrate-preselected HCT15 cells, whereas in control cells they are disperse as well as in SW480 control and butyrate-selected cells. D, HCT15 cells preselected by butyrate consume 20% less glucose titan control cells.
FIGURE 3.
HCT15 cells preselected with butyrate express more FoxM1 and membrane and metastases-associated proteins. SuperArray analysis for cell membrane and metastasis markers revealed that HCT15 cells preselected with butyric acid express higher levels of genes related to inhibition of glucose uptake, low differentiation, cell cycle and proliferation, and a more invasive (metastatic) behavior. These data were obtained from three independent experiments, with consistent results.
FIGURE 4.
HCT15 cells preselected with butyrate, _in vivo (_Balb/SCID mice), induce more vascularized and proliferative tumors, generating of a functional VEGF:KDR autocrine loop, and exhibited a higher capacity of homing in lungs. A, subcutaneous inoculation of HCT15 cells treated with butyric acid gave rise to significantly bigger tumors (p = 0.0097) than non-treated HCT15 cells, treatment of mice, inoculated with butyrate-preselected HCT15 cells, with IMC-1C11 blocks the augmented tumor growth; B, immunoreactivity for phospho-Histone3 showed that tumors developed from HCT15 cells treated with butyric acid are significantly more proliferative (p = 0.02) than tumors from non-treated HCT15 cells, treatment with IMC-1C11 also decreases proliferation; C, immunoreactivity for CD31 in subcutaneous tumors showed that tumors developed from HCT15 cells preselected with butyric acid are significantly more vascularized (p < 0.0001) than tumors from non-treated HCT15 cells, treatment with IMC-1C11 also decreases vascularization; D, H&E from lung sections (quantified in the plot) showed that mice injected with HCT15 cells preselected with butyric acid developed significantly more lung homing spots than control cells. These data were obtained from three independent experiments (6 mice/experiment/experimental condition), with consistent results. *, statistical significance.
FIGURE 5.
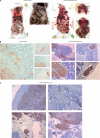
Xenografted orthotopic models in Balb/SCID mice showed that butyrate-preselected HCT15 cells are more invasive and metastastic than control cells. A, macroscopic observation showed that mice inoculated with control cells had only a tumor in the cecum, whereas mice inoculated with butyrate-preselected HCT15 cells had a local tumor and distant metastasis in the liver, kidney, and mediastinum; B, histological analysis showed that mice inoculated with control cells had a bigger tumor mass in the point of injection, in lamina propria and forming gland-like structures, whereas mice inoculated with butyrate-preselected HCT15 cells had a smaller tumor mass and an extended angio invasion; and C, mice inoculated with butyrate-preselected HCT15 cells presented liver, lung, and kidney metastasis and serous invasion of the spleen.
FIGURE 6.
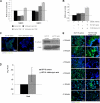
HCT15 cells preselected with butyrate exhibit a more invasive and motile phenotype. A, analysis of cell culture supernatants by gelatinolytic zymography showed that HCT15 cells after 120 h of butyric acid selection produce more matrix metalloproteinases 9 and 2 (MMP9 and MMP2) than those produced by control cells; B, transwell assay showed that HCT15 cells preselected with butyric acid migrate more in response to EGF (p < 0.04); C, butyrate-preselected HCT15 cells have reduced E-cadherin expression by immunofluorescense and Western blotting; D, RQ-PCR showed increased levels of mRNA from SNAIL in butyrate-preselected HCT15 cells; E, as shown by immunofluorescence butyrate-preselected HCT15 cells have increased expression of integrins related to invasion and migration (α2, α3, αv, and β3), and decreases the expression of integrins related to the maintenance of epithelium architecture (α5 and α6). These data were obtained from three independent experiments, with consistent results.
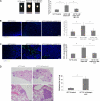
FIGURE 7.
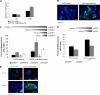
HCT15 cells preselected with butyrate have a more angiogenic phenotype. A, as determined by ELISA, butyrate-preselected HCT15 cells express more VEGF than control cells (p = 0.03); B, as determined by immunofluorescence, butyrate-preselected HCT15 cells express more KDR than control cells. These data were obtained from three independent experiments, with consistent results; C, luciferase reporter gene assay showed that the VEGF promoter sequence −131 to −1 is significantly more active (p < 0.02) than the other tested sequences in HCT15 cells, and deletion constructs pVEGF1, pVEGF2, and pVEGF4 are significantly stimulated in cells preselected by butyric acid (p = 0.041 for pVEGF1, p = 0.0073 for pVEGF2 and p = 0.0098 for pVEGF4); D, luciferase reporter gene assay showed that the KDR promoter sequence −446 to −1 is significantly more active (p = 0.046) than the sequence −796 to −1 in HCT15 cells, and both deletion constructs of KDR promoter are significantly stimulated in cells preselected by butyric acid (p = 0.0205 for pKDR1 and p = 0.0477 for pKDR2; E, as shown by immunofluorescence, butyrate-preselected HCT15 cells show low levels of FoxO1 and increased expression of FoxM1 transcription factor.
Similar articles
- The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation.
Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. Hinnebusch BF, et al. J Nutr. 2002 May;132(5):1012-7. doi: 10.1093/jn/132.5.1012. J Nutr. 2002. PMID: 11983830 - Butyrate, a dietary fiber derivative that improves irinotecan effect in colon cancer cells.
Encarnação JC, Pires AS, Amaral RA, Gonçalves TJ, Laranjo M, Casalta-Lopes JE, Gonçalves AC, Sarmento-Ribeiro AB, Abrantes AM, Botelho MF. Encarnação JC, et al. J Nutr Biochem. 2018 Jun;56:183-192. doi: 10.1016/j.jnutbio.2018.02.018. Epub 2018 Mar 3. J Nutr Biochem. 2018. PMID: 29587241 - GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon.
Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, Prasad PD, Ganapathy V. Thangaraju M, et al. Cancer Res. 2009 Apr 1;69(7):2826-32. doi: 10.1158/0008-5472.CAN-08-4466. Epub 2009 Mar 10. Cancer Res. 2009. PMID: 19276343 Free PMC article. - Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre.
Scharlau D, Borowicki A, Habermann N, Hofmann T, Klenow S, Miene C, Munjal U, Stein K, Glei M. Scharlau D, et al. Mutat Res. 2009 Jul-Aug;682(1):39-53. doi: 10.1016/j.mrrev.2009.04.001. Epub 2009 Apr 19. Mutat Res. 2009. PMID: 19383551 Review. - Dietary resistant starch and chronic inflammatory bowel diseases.
Jacobasch G, Schmiedl D, Kruschewski M, Schmehl K. Jacobasch G, et al. Int J Colorectal Dis. 1999 Nov;14(4-5):201-11. doi: 10.1007/s003840050212. Int J Colorectal Dis. 1999. PMID: 10647628 Review.
Cited by
- Unraveling FATP1, regulated by ER-β, as a targeted breast cancer innovative therapy.
Mendes C, Lopes-Coelho F, Ramos C, Martins F, Santos I, Rodrigues A, Silva F, André S, Serpa J. Mendes C, et al. Sci Rep. 2019 Oct 1;9(1):14107. doi: 10.1038/s41598-019-50531-3. Sci Rep. 2019. PMID: 31575907 Free PMC article. - Gut microbiota dysbiosis: The potential mechanisms by which alcohol disrupts gut and brain functions.
Chen G, Shi F, Yin W, Guo Y, Liu A, Shuai J, Sun J. Chen G, et al. Front Microbiol. 2022 Jul 29;13:916765. doi: 10.3389/fmicb.2022.916765. eCollection 2022. Front Microbiol. 2022. PMID: 35966709 Free PMC article. Review. - Manipulation of Gut Microbiota Using Acacia Gum Polysaccharide.
Rawi MH, Abdullah A, Ismail A, Sarbini SR. Rawi MH, et al. ACS Omega. 2021 Jul 2;6(28):17782-17797. doi: 10.1021/acsomega.1c00302. eCollection 2021 Jul 20. ACS Omega. 2021. PMID: 34308014 Free PMC article. - Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria.
LeBlanc JG, Chain F, Martín R, Bermúdez-Humarán LG, Courau S, Langella P. LeBlanc JG, et al. Microb Cell Fact. 2017 May 8;16(1):79. doi: 10.1186/s12934-017-0691-z. Microb Cell Fact. 2017. PMID: 28482838 Free PMC article. Review. - Establishment of a prognostic model for ovarian cancer based on mitochondrial metabolism-related genes.
Meng C, Sun Y, Liu G. Meng C, et al. Front Oncol. 2023 May 15;13:1144430. doi: 10.3389/fonc.2023.1144430. eCollection 2023. Front Oncol. 2023. PMID: 37256178 Free PMC article.
References
- Bugaut M. (1987) Comp. Biochem. Physiol. B 86, 439–472 - PubMed
- Roediger W. E. (1982) Gastroenterology 83, 424–429 - PubMed
- Wong J. M., de Souza R., Kendall C. W., Emam A., Jenkins D. J. (2006) J. Clin. Gastroenterol. 40, 235–243 - PubMed
- Leng S. L., Leeding K. S., Gibson P. R., Bach L. A. (2001) Carcinogenesis 22, 1625–1631 - PubMed
- Hinnebusch B. F., Meng S., Wu J. T., Archer S. Y., Hodin R. A. (2002) J. Nutr. 132, 1012–1017 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous