Cost and mortality prediction using polymerase chain reaction pathogen detection in sepsis: evidence from three observational trials - PubMed (original) (raw)
Comparative Study
doi: 10.1186/cc9294. Epub 2010 Oct 15.
Affiliations
- PMID: 20950442
- PMCID: PMC3219292
- DOI: 10.1186/cc9294
Comparative Study
Cost and mortality prediction using polymerase chain reaction pathogen detection in sepsis: evidence from three observational trials
Lutz E Lehmann et al. Crit Care. 2010.
Abstract
Introduction: Delays in adequate antimicrobial treatment contribute to high cost and mortality in sepsis. Polymerase chain reaction (PCR) assays are used alongside conventional cultures to accelerate the identification of microorganisms. We analyze the impact on medical outcomes and healthcare costs if improved adequacy of antimicrobial therapy is achieved by providing immediate coverage after positive PCR reports.
Methods: A mathematical prediction model describes the impact of PCR-based rapid adjustment of antimicrobial treatment. The model is applied to predict cost and medical outcomes for 221 sepsis episodes of 189 post-surgical and intensive care unit (ICU) sepsis patients with available PCR data from a prospective, observational trial of a multiplex PCR assay in five hospitals. While this trial demonstrated reduction of inadequate treatment days, data on outcomes associated with reduced inadequate initial antimicrobial treatment had to be obtained from two other, bigger, studies which involved 1,147 (thereof 316 inadequately treated) medical or surgical ICU patients. Our results are reported with the (5% to 95%) percentile ranges from Monte Carlo simulation in which the input parameters were randomly and independently varied according to their statistical characterization in the three underlying studies. The model allows predictions also for different patient groups or PCR assays.
Results: A total of 13.1% of PCR tests enabled earlier adequate treatment. We predict that cost for PCR testing (300 €/test) can be fully recovered for patients above 717 € (605 € to 1,710 €) daily treatment cost. A 2.6% (2.0 to 3.2%) absolute reduction of mortality is expected. Cost per incremental survivor calculates to 11,477 € (9,321 € to 14,977 €) and incremental cost-effectiveness ratio to 3,107 € (2,523 € to 4,055 €) per quality-adjusted life-year. Generally, for ICU patients with >25% incidence of inadequate empiric antimicrobial treatment, and at least 15% with a positive blood culture, PCR represents a cost-neutral adjunct method.
Conclusions: Rapid PCR identification of microorganisms has the potential to become a cost-effective component for managing sepsis. The prediction model tested with data from three observational trials should be utilized as a framework to deepen insights when integrating more complementary data associated with utilization of molecular assays in the management of sepsis.
Figures
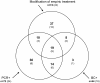
Figure 1
Modification of empiric antimicrobial treatment and microbiological characterization: Among 221 investigated sepsis episodes, 74 (33.5%) required modification of empiric antimicrobial treatment (upper circle). Positive blood cultures (lower right circle) triggered 27 (= 8 + 19) of these changes. Among 73 PCR+ episodes (lower left circle), 29 (= 10 + 19) allowed earlier adequate treatment (data from [17]). In brackets: Non-survivors within the respective groups.
Figure 2
Impact from PCR testing. Diagrams for estimating impact from PCR testing in sepsis, based on incidence of modification of initial antimicrobial treatment (x-axis) and share of episodes with positive blood culture (curves): A: Cost-neutral application of PCR is predicted if the mean daily treatment cost of those included in PCR testing exceeds the break-even value on the y-axis (A). Data point from our study: 717 €, at 20% BC+ (Table 2) and 33.5% modification of empiric treatment (= 74/221, Figure 1). B: Cost per incremental survivor is predicted as indicated on the y-axis (B). Data point from our study: 11,477 €, at 20% BC+ and 33.5% modification of empiric treatment. Figure 2 was calculated using equation 4 (A) and equation 5 (B) with substitution terms as given in Additional data file 2.
Figure 3
Predicted mortality reduction. Predicted mortality reduction if earlier adequate treatment is achieved in 29 of the 73 PCR+ episodes: A: Under adequate treatment, a 27.3% mortality is observed (= 12/(12 + 32)); IA: In the inadequately treated (IA) group, a mortality of 41.4% is observed (= 12/(12 + 17)); A*: A PCR+ based intervention should reduce the mortality in the former IA group according to equation 5 and the reduced relative risk of dying, RR† (from [3,4]). We predict that 5 of the 12 (Table 3) non-survivors might have survived if the PCR+ results were interventionally used. The resulting mortality of 26.0% (= (12 + 7)/73) is comparable to the mortality observed under adequate treatment (A).
Comment in
- PCR for the diagnosis of sepsis: hope or hype?
Schrenzel J. Schrenzel J. Crit Care. 2011 Jan 26;15(1):111. doi: 10.1186/cc9378. Crit Care. 2011. PMID: 21345277 Free PMC article.
Similar articles
- PCR for the diagnosis of sepsis: hope or hype?
Schrenzel J. Schrenzel J. Crit Care. 2011 Jan 26;15(1):111. doi: 10.1186/cc9378. Crit Care. 2011. PMID: 21345277 Free PMC article. - Potential clinical utility of polymerase chain reaction in microbiological testing for sepsis.
Lehmann LE, Alvarez J, Hunfeld KP, Goglio A, Kost GJ, Louie RF, Raglio A, Regueiro BJ, Wissing H, Stüber F. Lehmann LE, et al. Crit Care Med. 2009 Dec;37(12):3085-90. doi: 10.1097/CCM.0b013e3181b033d7. Crit Care Med. 2009. PMID: 19633541 - Economic aspects of severe sepsis: a review of intensive care unit costs, cost of illness and cost effectiveness of therapy.
Burchardi H, Schneider H. Burchardi H, et al. Pharmacoeconomics. 2004;22(12):793-813. doi: 10.2165/00019053-200422120-00003. Pharmacoeconomics. 2004. PMID: 15294012 Review. - [Cost-effectiveness analysis of schizophrenic patient care settings: impact of an atypical antipsychotic under long-acting injection formulation].
Llorca PM, Miadi-Fargier H, Lançon C, Jasso Mosqueda G, Casadebaig F, Philippe A, Guillon P, Mehnert A, Omnès LF, Chicoye A, Durand-Zaleski I. Llorca PM, et al. Encephale. 2005 Mar-Apr;31(2):235-46. doi: 10.1016/s0013-7006(05)82390-5. Encephale. 2005. PMID: 15959450 Review. French.
Cited by
- PCR for the diagnosis of sepsis: hope or hype?
Schrenzel J. Schrenzel J. Crit Care. 2011 Jan 26;15(1):111. doi: 10.1186/cc9378. Crit Care. 2011. PMID: 21345277 Free PMC article. - Design and Construction of a Single-Tube, LATE-PCR, Multiplex Endpoint Assay with Lights-On/Lights-Off Probes for the Detection of Pathogens Associated with Sepsis.
Carver-Brown RK, Reis AH Jr, Rice LM, Czajka JW, Wangh LJ. Carver-Brown RK, et al. J Pathog. 2012;2012:424808. doi: 10.1155/2012/424808. Epub 2012 Dec 26. J Pathog. 2012. PMID: 23326668 Free PMC article. - Structured oligonucleotides for target indexing to allow single-vessel PCR amplification and solid support microarray hybridization.
Girard LD, Boissinot K, Peytavi R, Boissinot M, Bergeron MG. Girard LD, et al. Analyst. 2015 Feb 7;140(3):912-21. doi: 10.1039/c4an01352b. Analyst. 2015. PMID: 25489607 Free PMC article. - Clinical evaluation of droplet digital pcr for suspected ascites infection in patients with liver cirrhosis.
Han J, Wei FL, Wu HX, Guo LY, Guo S, Han Y, Sun YN, Hou W, Hu ZJ. Han J, et al. Hepatol Int. 2024 Aug;18(4):1249-1260. doi: 10.1007/s12072-024-10669-3. Epub 2024 Apr 29. Hepatol Int. 2024. PMID: 38683274 - Ion mobility spectrometry for microbial volatile organic compounds: a new identification tool for human pathogenic bacteria.
Jünger M, Vautz W, Kuhns M, Hofmann L, Ulbricht S, Baumbach JI, Quintel M, Perl T. Jünger M, et al. Appl Microbiol Biotechnol. 2012 Mar;93(6):2603-14. doi: 10.1007/s00253-012-3924-4. Epub 2012 Feb 12. Appl Microbiol Biotechnol. 2012. PMID: 22327321 Free PMC article.
References
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. - DOI - PubMed