Lifespan extension by preserving proliferative homeostasis in Drosophila - PubMed (original) (raw)
Lifespan extension by preserving proliferative homeostasis in Drosophila
Benoît Biteau et al. PLoS Genet. 2010.
Abstract
Regenerative processes are critical to maintain tissue homeostasis in high-turnover tissues. At the same time, proliferation of stem and progenitor cells has to be carefully controlled to prevent hyper-proliferative diseases. Mechanisms that ensure this balance, thus promoting proliferative homeostasis, are expected to be critical for longevity in metazoans. The intestinal epithelium of Drosophila provides an accessible model in which to test this prediction. In aging flies, the intestinal epithelium degenerates due to over-proliferation of intestinal stem cells (ISCs) and mis-differentiation of ISC daughter cells, resulting in intestinal dysplasia. Here we show that conditions that impair tissue renewal lead to lifespan shortening, whereas genetic manipulations that improve proliferative homeostasis extend lifespan. These include reduced Insulin/IGF or Jun-N-terminal Kinase (JNK) signaling activities, as well as over-expression of stress-protective genes in somatic stem cell lineages. Interestingly, proliferative activity in aging intestinal epithelia correlates with longevity over a range of genotypes, with maximal lifespan when intestinal proliferation is reduced but not completely inhibited. Our results highlight the importance of the balance between regenerative processes and strategies to prevent hyperproliferative disorders and demonstrate that promoting proliferative homeostasis in aging metazoans is a viable strategy to extend lifespan.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
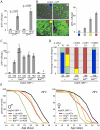
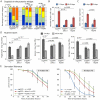
Figure 1. Intestinal homeostasis and tissue regeneration is critical for normal lifespan.
A. Age-related increase in the frequency of pH3+ cells in the aging intestine of wild-type flies (Average and SEM is shown). ISC over-proliferation is accelerated at higher temperature (29°C). Intestines were dissected at the indicated age and phosphorylated Histone H3 was detected by immunohistochemistry. B. The size of GFP+ cell clusters can be used to evaluate dysplasia in esgGal4, UAS-GFP flies (see also Text S1). The 4 categories defined visually in the panels on the left correlate with the frequency of pH3+ cells in the gut (right). C. Activation of JNK and IIS pathways in ISC (esg>Hep and esg>inR respectively) induces over-proliferation as early as 5 days, while inhibition of JNK (esg>BskRNAi) prevents tissue regeneration as shown by much reduced frequency of pH3+ cells. The TARGET system was used to prevent developmental effects of esg-driven transgenes expression (Genotypes: w 1118;esgGal4,UAS-GFP/+;tubGal80ts, w 1118;esgGal4,UAS-GFP/UAS-HepWT;tG80ts, w 1118;esgGal4,UAS-GFP/UAS-InRWT;tG80ts, and w 1118;esgGal4,UAS-GFP/UAS-BskRNAi;tG80ts). Flies were reared at 18°C and then aged at 29°C to restrict expression of transgenes to adulthood. Averages and SEM are shown. * p<0.001 compared to Control at 5 days; # p<0.001 compared to Control at 18 days using Student's t-test. D. Intestinal dysplasia in the flies described above was monitored using the method described in Figure 1B (see also Text S1) after 18 days. Activation of JNK and IIS pathways causes accelerated dysplasia, reduction of JNK signaling leads to a complete prevention of tissue regeneration. p-value from Pearson XiSquare test. E. Flies with impaired intestinal homeostasis and tissue regeneration are short-lived. The mortality of the flies described above was recorded at 29°C. Detailed lifespan analysis is shown in Table S1.
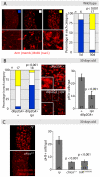
Figure 2. Reduced IIS activity delays tissue degeneration in the intestine.
A. Evaluation of intestinal dysplasia in aging wild-type flies. BrdU incorporation identifies proliferating cells (nuclear, red), while immunohistochemistry with anti-Armadillo antibodies detects changes in epithelial structure (membrane, red). Flies were aged at 25°C, and fed BrdU for 48 hrs. B. Reducing systemic insulin signaling by ablation of Insulin Producing Cells (IPCs) delays aging-associated dysplasia. Representative pictures of the midgut from aging (30 days old at 25°C) control flies (w 1118;dilp2Gal4>+) and flies with ablated IPC's (w 1118;dilp2Gal4>rpr) are shown in the center. Scoring was performed based on the classification shown in B. Significant delay of dysplasia in dilp2Gal4>rpr can be observed compared to dilp2G>+ controls (left panel, Pearson Xi Square test). This correlates with reduced numbers of pH3+ cells in dilp2Gal4>rpr (Average and SEM; Student's t-test). C. Frequency of pH3+ cells in aging chico1/1 homozygotes and InrE19/05545 compared to isogenic wild-type controls (ry506) (Average and SEM, Student's t- test). Representative BrdU/Armadillo-stained midguts from aging chico1 mutant flies and sibling controls (ry) are shown on the left.
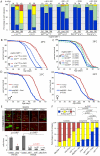
Figure 3. Strong reduction of insulin signaling in the somatic stem cell lineages delays age-related dysplasia and shortens lifespan.
A. Intestinal degeneration was monitored in aging control flies (w 1118;esgGal4,GFP;Gal80ts and y 1 w 1;esgGal4,GFP;Gal80ts) and flies with impaired insulin signaling activity in ISCs (w 1118;esgGal4,GFP;Gal80ts/UAS-AktRNAi, y 1 w 1;esgGal4,GFP;Gal80ts/UAS-Dp110DN, y 1 w 1;esgGal4,GFP;Gal80ts/UAS-InRDN, y 1 w 1;esgGal4,GFP;Gal80ts/UAS-Dp110DN, y 1 w 1;esgGal4,GFP/UAS-Foxo;Gal80ts). Strong inhibition of IIS in ISC prevents age-related intestinal dysplasia. p-value from Pearson XiSquare test. B,C. Flies with impaired intestinal homeostasis and tissue regeneration are short-lived. The mortality of the flies described above was recorded at 29°C. Detailed lifespan analysis is shown in Table S2. D. EsgGal4 was used to express Foxo in the ISC lineage. Fly lines were backcrossed into the w1118 background (10 generations) and sibling populations derived from crosses of w 1118;esgGal4/+ females with y 1 w 1;UAS-Foxo/UAS-Foxo males were compared. Flies were reared at 18°C to minimize driver activity during development, and adults were maintained at 25°C. Lifespan is significantly shortened in flies expressing Foxo under the control of esgGal4. A detailed analysis of the mortality, as well as the mortality of isogenic controls (w 1118/y 1 w 1), flies is shown in Table S3. E. Growth of InR and chico1 homozygous mutant ISC clones and clones over-expressing Foxo in the intestinal epithelium. Clones were induced using the MARCM system by heat-shock at three days of age and clone size was evaluated at 7 days after heat shock. chico1 clones exhibit reduced, but not absent growth, while InR mutant clones and Foxo over-expressing clones remain mostly single cells. Representative images are shown in top panels (Green: GFP; Red: Armadillo/Prospero). Clone size quantification is shown in lower graphic (Averages and SEM). p-values from Student's t-test. InR homozygous mutant clones and clones over-expressing Foxo grow significantly less than chico1 mutant clones (p-values in red). F. Comparison of the proliferation rate in the intestine of controls, long-lived and short-lived flies, after 30 days at 25°C, suggesting that long-lived mutants achieve the proper balance between tissue dysplasia and absence of regeneration. p-value from Pearson XiSquare test.
Figure 4. Moderate inhibition of IIS and JNK pathways in somatic stem cell lineages extends lifespan.
A. The 5961GS driver is expressed in the intestine and responsive to RU486. GFP can be detected in the posterior midgut of 5961GS>GFP flies after RU486 exposure, no GFP is detected when flies are kept on control food. B. In the intestine, the activity of the 5961GS driver is restricted to ISC and EB. Only LacZ-positive cells express GFP in 5961GS>GFP/esg-LacZ flies. The expression of the reporter esg-LacZ identifies ISC and EB, immunostaining against prospero identifies EE. C. Western-blot analysis of total extract from dissected guts shows that GFP can be detected in the intestine of 5961GS>GFP flies after RU486 exposure. However, the expression level remains much lower than in the intestine of esgGal4>GFP flies. D–J. Moderate reduction of the IIS (F, G, I) and JNK (H, J) pathways using 5961GS extends lifespan. The mortality of sibling flies of the indicated genotypes placed on control food (-RU486) or food supplemented with RU486 (+RU486) was compared at 25°C. The treatment has minimal effect on the longevity of control flies (5961GS,UAS-GFP>+ in w 1118 and OreR background), but causes significant increase in longevity of flies with reduced IIS and JNK pathways (5961GS,UAS-GFP>UAS-InRDN, 5961GS,UAS-GFP>UAS-Dp110DN, 5961GS,UAS-GFP>UAS-AktRNAi, 5961GS,UAS-GFP>UAS-BskDN, 5961GS,UAS-GFP>UAS-BskRNAi). The relative extension of the median lifespan is shown for each genetic condition. For each condition, the reduction of ISC proliferation by the treatment was confirmed, as measured by the number of pH3+ cells in the intestinal epithelium, in 50 to 70 days old females (n>12 guts; Averages and SEM; p-values from Student's t-test * p<0.05, ** p<0.01). K. Summary of lifespan statistics including mean and median lifespan (days) for all conditions. Detailed lifespan analysis is shown in Table S4.
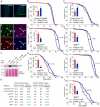
Figure 5. Overexpression of stress-protective genes in the somatic stem cell lineages delays intestinal degeneration and limits metabolic decay.
A. Overexpression of Jafrac1 or Hsp68 delays age-related loss of intestinal architecture. Intestinal degeneration in aging (3, 35, and 50 days) control flies (esgGFP>+) and flies overexpressing cytoprotective genes in the ISCs (esgGFP>Jafrac1 and esgGFP>Hsp68) was scored in the posterior midgut. p- values from Pearson Xi Square test. B. Overexpression of Jafrac1 or Hsp68 under the control of esgGal4 also limits the increase in the frequency of pH3+ cells in aging intestines (Averages and SEM; Student's t-test). C. Dl expression relative to rp49 in the aging intestine measured by real-time RT-PCR (Averages and SEM; Student's t-test). D. Over-expression of Jafrac1 or Hsp68 delays age-related changes in nutrient levels. Triglycerides, free glucose and glycogens were measured in young (3–4 days old) or old (40–50 days old) flies. Concentration is shown as mg nutrient per mg fresh fly. The number at the bottom of each bar represents the number of samples. All error bars represent standard deviation, p-value from Student's t-test. E. Jafrac1 and Hsp68 expression in ISCs increases starvation tolerance in old flies. Wet starvation resistance was determined in the indicated populations of flies aged for three days (left) or for 30 days (right).
Figure 6. Over-expression of stress-protective genes in the somatic stem cell lineages extends lifespan.
A. Survival curves of esgGFP>Jafrac1 and esgGFP>Hsp68 flies compared to their respective wild type isogenic controls. UAS lines were backcrossed 10 generation into w 1118 background. Wild type and UAS siblings were crossed to esgGFP and mortality of the progeny was recorded at 25°C. B. Summary of lifespan statistics including mean and median lifespan (days). C. Over-expression of Jafrac1 and Hsp68 using the 5961GS driver moderately extends lifespan. The mortality of sibling flies (5961GS,GFP>UAS-Jafrac1,UAS-Hsp68) placed on control food or food supplemented with RU486 was compared at 25°C. Due to the weak activity of the 5961GS driver, both UAS-Jafrac1 and UAS-Hsp68 transgenes were combined to observe a significant effect. The relative extension of the median lifespan is shown for all curves. Detailed lifespan analysis is shown in Table S5 and S6.
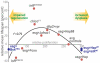
Figure 7. A model for the impact of regenerative capacity on lifespan.
Genetic conditions that moderately decrease ISC proliferation (thus limiting dysplasia) are associated with increased lifespan, while strong repression of ISC proliferation is deleterious for regeneration and shortens lifespan. The association of lifespan and regenerative capacity of the intestine in aging flies is illustrated by comparing ISC proliferation rates and lifespan. Relative ISC proliferation rates were calculated for each genotype using the three categories defined in Figure 3F (low, intermediate and high frequencies of pH3+ cells) and the following formula: × = (proportion in cat.1) + 2*(proportion in cat.2) + 3*(proportion in cat.3). This model includes lifespan and intestinal proliferation analysis from experiments conducted at 25°C (red dots) or 29°C (blue dots). The relative proliferation data is from this study. These data are plotted against lifespan changes in the respective genotypes relative to corresponding isogenic controls, from this work and from published studies , , . Polynomial regression curve (3rd degree) was fitted using Excel.
Similar articles
- βν integrin inhibits chronic and high level activation of JNK to repress senescence phenotypes in Drosophila adult midgut.
Okumura T, Takeda K, Taniguchi K, Adachi-Yamada T. Okumura T, et al. PLoS One. 2014 Feb 20;9(2):e89387. doi: 10.1371/journal.pone.0089387. eCollection 2014. PLoS One. 2014. PMID: 24586740 Free PMC article. - JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut.
Biteau B, Hochmuth CE, Jasper H. Biteau B, et al. Cell Stem Cell. 2008 Oct 9;3(4):442-55. doi: 10.1016/j.stem.2008.07.024. Cell Stem Cell. 2008. PMID: 18940735 Free PMC article. - The role of p38b MAPK in age-related modulation of intestinal stem cell proliferation and differentiation in Drosophila.
Park JS, Kim YS, Yoo MA. Park JS, et al. Aging (Albany NY). 2009 May 21;1(7):637-51. doi: 10.18632/aging.100054. Aging (Albany NY). 2009. PMID: 20157545 Free PMC article. - Promoting longevity by maintaining metabolic and proliferative homeostasis.
Wang L, Karpac J, Jasper H. Wang L, et al. J Exp Biol. 2014 Jan 1;217(Pt 1):109-18. doi: 10.1242/jeb.089920. J Exp Biol. 2014. PMID: 24353210 Free PMC article. Review. - JNK Signaling in Drosophila Aging and Longevity.
Gan T, Fan L, Zhao L, Misra M, Liu M, Zhang M, Su Y. Gan T, et al. Int J Mol Sci. 2021 Sep 6;22(17):9649. doi: 10.3390/ijms22179649. Int J Mol Sci. 2021. PMID: 34502551 Free PMC article. Review.
Cited by
- BNIP3 as a new tool to promote healthy brain aging.
Neikirk K, Marshall AG, Santisteban MM, Hinton A Jr. Neikirk K, et al. Aging Cell. 2024 Feb;23(2):e14042. doi: 10.1111/acel.14042. Epub 2023 Nov 29. Aging Cell. 2024. PMID: 38030595 Free PMC article. Review. - A virus-acquired host cytokine controls systemic aging by antagonizing apoptosis.
Mlih M, Khericha M, Birdwell C, West AP, Karpac J. Mlih M, et al. PLoS Biol. 2018 Jul 23;16(7):e2005796. doi: 10.1371/journal.pbio.2005796. eCollection 2018 Jul. PLoS Biol. 2018. PMID: 30036358 Free PMC article. Updated. - Heterochromatin Protein 1 (HP1) inhibits stem cell proliferation induced by ectopic activation of the Jak/STAT pathway in the Drosophila testis.
Loza-Coll MA, Petrossian CC, Boyle ML, Jones DL. Loza-Coll MA, et al. Exp Cell Res. 2019 Apr 15;377(1-2):1-9. doi: 10.1016/j.yexcr.2019.02.024. Epub 2019 Feb 25. Exp Cell Res. 2019. PMID: 30817931 Free PMC article. - The septate junction component bark beetle is required for Drosophila intestinal barrier function and homeostasis.
Hodge RA, Ghannam M, Edmond E, de la Torre F, D'Alterio C, Kaya NH, Resnik-Docampo M, Reiff T, Jones DL. Hodge RA, et al. iScience. 2023 May 19;26(6):106901. doi: 10.1016/j.isci.2023.106901. eCollection 2023 Jun 16. iScience. 2023. PMID: 37332603 Free PMC article. - Antimicrobial peptides extend lifespan in Drosophila.
Loch G, Zinke I, Mori T, Carrera P, Schroer J, Takeyama H, Hoch M. Loch G, et al. PLoS One. 2017 May 17;12(5):e0176689. doi: 10.1371/journal.pone.0176689. eCollection 2017. PLoS One. 2017. PMID: 28520752 Free PMC article.
References
- Guarente L, Picard F. Calorie restriction—the SIR2 connection. Cell. 2005;120:473–482. - PubMed
- Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. - PubMed
- Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. - PubMed
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous