Nrf2-mediated liver protection by sauchinone, an antioxidant lignan, from acetaminophen toxicity through the PKCδ-GSK3β pathway - PubMed (original) (raw)
Nrf2-mediated liver protection by sauchinone, an antioxidant lignan, from acetaminophen toxicity through the PKCδ-GSK3β pathway
Hee Yeon Kay et al. Br J Pharmacol. 2011 Aug.
Abstract
BACKGROUND AND PURPOSE Sauchinone, an antioxidant lignan, protects hepatocytes from iron-induced toxicity. This study investigated the protective effects of sauchinone against acetaminophen (APAP)-induced toxicity in the liver and the role of nuclear factor erythroid-2-related factor-2 (Nrf2) in this effect. EXPERIMENTAL APPROACH Blood biochemistry and histopathology were assessed in mice treated with APAP or APAP + sauchinone. The levels of mRNA and protein were measured using real-time PCR assays and immunoblottings. KEY RESULTS Sauchinone ameliorated liver injury caused by a high dose of APAP. This effect was prevented by a deficiency of Nrf2. Sauchinone treatment induced modifier subunit of glutamate-cysteine ligase, NAD(P)H:quinone oxidoreductase-1 (NQO1) and heat shock protein 32 in the liver, which was abolished by Nrf2 deficiency. In a hepatocyte model, sauchinone activated Nrf2, as evidenced by the increased nuclear accumulation of Nrf2, the induction of NQO1-antioxidant response element reporter gene, and glutamate-cysteine ligase and NQO1 protein induction, which contributed to the restoration of hepatic glutathione content. Consistently, treatment of sauchinone enhanced Nrf2 phosphorylation with a reciprocal decrease in its interaction with Kelch-like ECH-associated protein-1. Intriguingly, sauchinone activated protein kinase C-δ (PKCδ), which led to Nrf2 phosphorylation. In addition, it increased the inhibitory phosphorylation of glycogen synthase kinase-3β (GSK3β), derepressing Nrf2 activity, which was supported by the reversal of sauchinone's activation of Nrf2 by an activated mutant of GSK3β. Moreover, phosphorylation of GSK3β by sauchinone depended on PKCδ activation. CONCLUSION AND IMPLICATIONS Our results demonstrate that sauchinone protects the liver from APAP-induced toxicity by activating Nrf2, and this effect is mediated by PKCδ activation, which induces inhibitory phosphorylation of GSK3β.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
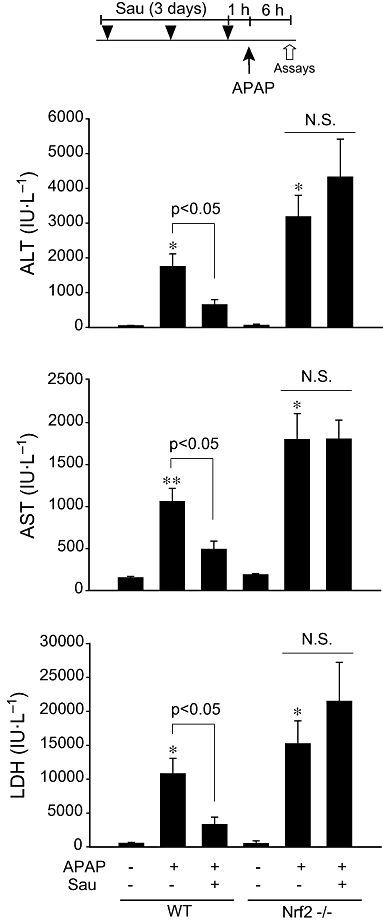
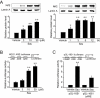
Figure 1
The effect of sauchinone (Sau) on acetaminophen (APAP)-induced liver injury. Male wild-type (WT) or Nrf2 knockout mice that had been treated with Sau (30 mg·kg−1·day−1, p.o., for 3 days) were administered a single dose of APAP (500 mg·kg−1, i.p.). The animals were killed 6 h after APAP treatment. The activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were measured in the plasma samples. N.S., not significant.
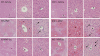
Figure 2
Haematoxylin and eosin staining of the liver. Mice were treated with acetaminophen (APAP) or APAP + sauchinone (Sau), as described in the legend to Figure 1 (n = 6 each). Arrows indicate degeneration and necrosis of the hepatocytes. C, central vein; P, portal space; *, haemorrhage; scale bars = 200 µm in left column and 50 µm in middle and right columns; Low-power view (left column) and high-power view of the central and portal area (right column). Nrf2, nuclear factor erythroid-2-related factor-2; WT, wild-type.
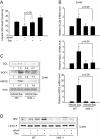
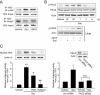
Figure 3
The effects of sauchinone (Sau) on hepatic glutathione (GSH) contents and glutamate-cysteine ligase (GCL), NAD(P)H:quinone oxidoreductase-1 (NQO1) and heat shock protein 32 (HSP32) expression. (A) Hepatic GSH contents. Mice were treated with acetaminophen (APAP) or APAP + Sau, as described in the legend to Figure 1 (n = 6 each). (B) Real-time PCR assays for modifier subunit of glutamate-cysteine ligase (GCLM), NQO1 and HSP32. Sau (30 mg·kg−1·day−1) was administered to wild-type (WT) or nuclear factor erythroid-2-related factor-2 (Nrf2) knockout mice for 3 days, and the liver samples were collected 6 h after last injection of Sau (n = 6 each). (C) Immunoblottings for GCL, NQO1 and HSP32 in the liver homogenates. Values indicate scanning densitometric results. Representative data from six samples. (D) Immunoblottings for Nrf2 in the nuclear fractions of liver homogenates. Representative data from six samples.
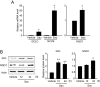
Figure 4
Induction of glutamate-cysteine ligase (GCL) and NAD(P)H:quinone oxidoreductase-1 (NQO1) by sauchinone. (A) Real-time PCR assays. The expression of nuclear factor erythroid-2-related factor-2 (Nrf2) target genes was monitored in HepG2 cells using real-time PCR assays, in which the mRNA level of GAPDH was used as a reference for data normalization. Cells were treated with vehicle or 30 µM sauchinone (Sau) for 12 h and subjected to the preparation of mRNA, from which cDNA was synthesized by reverse transcriptase. Fold changes were calculated by correlation coefficients of crossing point (Cp) for triplicate PCR results. (B) Immunoblottings for GCL and NQO1. The proteins of interest were immunoblotted in the lysates of HepG2 cells that had been treated with vehicle or 30 µM sauchinone for 24 h. GCLC, catalytic subunit of glutamate-cysteine ligase; GCLM, modifier subunit of glutamate-cysteine ligase.
Figure 5
The effects of sauchinone (Sau) on nuclear factor erythroid-2-related factor-2 (Nrf2) activation and antioxidant response element (ARE) reporter gene induction. (A) Nuclear accumulation of Nrf2. Nrf2 contents were measured in the nuclear fractions of HepG2 cells treated with vehicle or Sau as indicated above. (B) NAD(P)H:quinone oxidoreductase-1 (NQO1) ARE reporter assays. Luciferase activity was measured on the lysates of cells treated with 3–30 µM sauchinone for 24 h following transfection with a NQO1-ARE construct. (C) The effect of Sau on the induction of luciferase activity from pGL-1651 or pGL-1651-ΔARE. Luciferase activity was measured in cells that had been transfected with pGL-1651 or pGL-1651-ΔARE, and exposed to vehicle or 30 µM Sau for 24 h. The values represent the mean ± SE of four separate experiments (significant compared with control, **P < 0.01; control = 1). C/EBP, CCAAT/enhancer-binding protein; HRE, HNF response element.
Figure 6
Sauchinone (Sau) activation of protein kinase C δ (PKCδ) and PKCδ-dependent nuclear factor erythroid-2-related factor-2 (Nrf2) activity. (A) Nrf2 phosphorylation with a reciprocal decrease in the interaction between Nrf2 and Kelch-like ECH-associated protein-1 (Keap1). HepG2 cells were treated with vehicle or Sau for 3 h. Nrf2 immunoprecipitates were subjected to immunoblottings for phosphorylated serine and Keap1. Results were confirmed by repeated experiments. _tert_-butylhydroquinone, t-BHQ. (B) The effect of Sau treatment on the phosphorylation of PKCδ. Immunoblottings were performed on the lysates of HepG2 cells treated with 30 µM Sau for 10 min–6 h (upper), or the livers of mice treated with 30 mg·kg−1 Sau and/or 500 mg·kg−1 APAP for 6 h (lower). (C) Inhibition by phorbol 12-myristate 13-acetate (PMA) or rottlerin of the ability of Sau to activate Nrf2. Nrf2 was immunoblotted in the nuclear fractions of cells treated with 30 µM Sau alone or in combination with 1 µM PMA or 2 µM rottlerin for 3 h. Luciferase activity was measured on the lysates of pGL-1651-transfected cells exposed to Sau alone or in combination with PMA or rottlerin for 24 h (left). In addition, luciferase activity was similarly determined after siRNA transfection (right). Data represent the mean ± SE of 4 separate experiments (significant compared with the respective control, *P < 0.05, **P < 0.01).
Figure 7
Increase in GSK3β phosphorylation by sauchinone (Sau). (A) Immunoblottings for glycogen synthase kinase 3β (GSK3β). Ser9-phosphorylated or total GSK3β was immunochemically measured on the lysates of HepG2 cells treated with 30 µM Sau for 10 min–6 h (upper), or on the homogenates of livers of mice treated with 30 mg·kg−1 Sau and/or 500 mg·kg−1 acetaminophen (APAP) for 6 h (lower). Results were confirmed in three separate experiments. (B) Reversal by ΔSer9GSK3β (CA-GSK3β) of the ability of Sau to activate nuclear factor erythroid-2-related factor-2 (Nrf2). Nrf2 was immunoblotted in the lysates of mock- or ΔSer9GSK3β-transfected cells treated with vehicle or Sau for 3 h. Lamin A immunoblotting verified equal loading and the purity of nuclear proteins (upper). Luciferase activity was measured on the lysates of cells that had been transfected with pGL-1651 in combination with ΔSer9GSK3β for 24 h, followed by Sau treatment (lower). (C) Inhibition of Sau-induced GSK3β phosphorylation by protein kinase C δ (PKCδ) inhibition. The proteins of interest were immunoblotted on the lysates of cells treated with vehicle, Sau, Sau + rottlerin for 1 h. Similarly, the proteins were assessed after transfection with non-targeting siRNA (con siRNA) or siRNA directed against PKCδ. Results were confirmed by three repeated experiments. (D) Immunoprecipitation and immunoblot assays. Interaction between Kelch-like ECH-associated protein-1 (Keap1) and Nrf2 was measured in mock- or CA-GSK3β-transfected HepG2 cells that had been treated with 5 µM rottlerin for 1 h and continuously exposed to vehicle or Sau for 3 h. Nrf2 immunoprecipitates were subjected to immunoblottings for Keap1. Results were confirmed by repeated experiments. (E) Immunoblottings for p-PKCδ or p-GSK3β (Ser9) on the lysates of cells treated with 1 µM OSU03012 for 1 h and continuously incubated with Sau for 1 h. (F) A schematic diagram illustrating the proposed mechanism by which sauchinone protects the liver against APAP-induced toxicity. GCL, glutamate-cysteine ligase; GSH, glutathione; NAPQI, N-acetyl-p-benzoquinoneimine; NQO1, NAD(P)H:quinone oxidoreductase-1.
Similar articles
- Efficacy of sauchinone as a novel AMPK-activating lignan for preventing iron-induced oxidative stress and liver injury.
Kim YW, Lee SM, Shin SM, Hwang SJ, Brooks JS, Kang HE, Lee MG, Kim SC, Kim SG. Kim YW, et al. Free Radic Biol Med. 2009 Oct 1;47(7):1082-92. doi: 10.1016/j.freeradbiomed.2009.07.018. Epub 2009 Jul 17. Free Radic Biol Med. 2009. PMID: 19616619 - Corilagin alleviates acetaminophen-induced hepatotoxicity via enhancing the AMPK/GSK3β-Nrf2 signaling pathway.
Lv H, Hong L, Tian Y, Yin C, Zhu C, Feng H. Lv H, et al. Cell Commun Signal. 2019 Jan 10;17(1):2. doi: 10.1186/s12964-018-0314-2. Cell Commun Signal. 2019. PMID: 30630510 Free PMC article. - Schisandrol B protects against acetaminophen-induced acute hepatotoxicity in mice via activation of the NRF2/ARE signaling pathway.
Jiang YM, Wang Y, Tan HS, Yu T, Fan XM, Chen P, Zeng H, Huang M, Bi HC. Jiang YM, et al. Acta Pharmacol Sin. 2016 Mar;37(3):382-9. doi: 10.1038/aps.2015.120. Epub 2016 Jan 25. Acta Pharmacol Sin. 2016. PMID: 26806302 Free PMC article. - Recommendations for the use of the acetaminophen hepatotoxicity model for mechanistic studies and how to avoid common pitfalls.
Jaeschke H, Adelusi OB, Akakpo JY, Nguyen NT, Sanchez-Guerrero G, Umbaugh DS, Ding WX, Ramachandran A. Jaeschke H, et al. Acta Pharm Sin B. 2021 Dec;11(12):3740-3755. doi: 10.1016/j.apsb.2021.09.023. Epub 2021 Sep 30. Acta Pharm Sin B. 2021. PMID: 35024303 Free PMC article. Review.
Cited by
- Therapeutic Potential of Plants and Plant Derived Phytochemicals against Acetaminophen-Induced Liver Injury.
Subramanya SB, Venkataraman B, Meeran MFN, Goyal SN, Patil CR, Ojha S. Subramanya SB, et al. Int J Mol Sci. 2018 Nov 28;19(12):3776. doi: 10.3390/ijms19123776. Int J Mol Sci. 2018. PMID: 30486484 Free PMC article. Review. - Targeting Glycogen Synthase Kinase-3β for Therapeutic Benefit against Oxidative Stress in Alzheimer's Disease: Involvement of the Nrf2-ARE Pathway.
Kanninen K, White AR, Koistinaho J, Malm T. Kanninen K, et al. Int J Alzheimers Dis. 2011;2011:985085. doi: 10.4061/2011/985085. Epub 2011 May 2. Int J Alzheimers Dis. 2011. PMID: 21629716 Free PMC article. - CD147 confers temozolomide resistance of glioma cells via the regulation of β-TrCP/Nrf2 pathway.
Bu X, Qu X, Guo K, Meng X, Yang X, Huang Q, Dou W, Feng L, Wei X, Gao J, Sun W, Chao M, Han L, Hu Y, Shen L, Zhang J, Wang L. Bu X, et al. Int J Biol Sci. 2021 Jul 13;17(12):3013-3023. doi: 10.7150/ijbs.60894. eCollection 2021. Int J Biol Sci. 2021. PMID: 34421346 Free PMC article. - Naturally Occurring Nrf2 Activators: Potential in Treatment of Liver Injury.
Jadeja RN, Upadhyay KK, Devkar RV, Khurana S. Jadeja RN, et al. Oxid Med Cell Longev. 2016;2016:3453926. doi: 10.1155/2016/3453926. Epub 2016 Dec 22. Oxid Med Cell Longev. 2016. PMID: 28101296 Free PMC article. Review. - Therapeutic targeting of GSK3β enhances the Nrf2 antioxidant response and confers hepatic cytoprotection in hepatitis C.
Jiang Y, Bao H, Ge Y, Tang W, Cheng D, Luo K, Gong G, Gong R. Jiang Y, et al. Gut. 2015 Jan;64(1):168-79. doi: 10.1136/gutjnl-2013-306043. Epub 2014 May 8. Gut. 2015. PMID: 24811996 Free PMC article.
References
- Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, Yamamoto M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. - PubMed
- Cho HY, Jedlicka AE, Reddy SP, Zhang LY, Kensler TW, Kleeberger SR. Linkage analysis of susceptibility to hyperoxia. Nrf2 is a candidate gene. Am J Respir Cell Mol Biol. 2002;26:42–51. - PubMed
- Chung BS, Shin MG. Dictionary of Korean Folk Medicine. Seoul: Young Lim Sa; 1990. pp. 813–814.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous