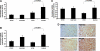Disruption of hypoxia-inducible transcription factor-prolyl hydroxylase domain-1 (PHD-1-/-) attenuates ex vivo myocardial ischemia/reperfusion injury through hypoxia-inducible factor-1α transcription factor and its target genes in mice - PubMed (original) (raw)
Disruption of hypoxia-inducible transcription factor-prolyl hydroxylase domain-1 (PHD-1-/-) attenuates ex vivo myocardial ischemia/reperfusion injury through hypoxia-inducible factor-1α transcription factor and its target genes in mice
Ram Sudheer Adluri et al. Antioxid Redox Signal. 2011.
Abstract
Hypoxia-inducible transcription factor (HIF)-prolyl hydroxylases domain (PHD-1-3) are oxygen sensors that regulate the stability of the HIFs in an oxygen-dependent manner. Suppression of PHD enzymes leads to stabilization of HIFs and offers a potential treatment option for many ischemic disorders, such as peripheral artery occlusive disease, myocardial infarction, and stroke. Here, we show that homozygous disruption of PHD-1 (PHD-1(-/-)) could facilitate HIF-1α-mediated cardioprotection in ischemia/reperfused (I/R) myocardium. Wild-type (WT) and PHD-1(-/-) mice were randomized into WT time-matched control (TMC), PHD-1(-/-) TMC (PHD1TMC), WT I/R, and PHD-1(-/-) I/R (PHD1IR). Isolated hearts from each group were subjected to 30 min of global ischemia followed by 2 h of reperfusion. TMC hearts were perfused for 2 h 30 min without ischemia. Decreased infarct size (35%±0.6% vs. 49%±0.4%) and apoptotic cardiomyocytes (106±13 vs. 233±21 counts/100 high-power field) were observed in PHD1IR compared to wild-type ischemia/reperfusion (WTIR). Protein expression of HIF-1α was significantly increased in PHD1IR compared to WTIR. mRNA expression of β-catenin (1.9-fold), endothelial nitric oxide synthase (1.9-fold), p65 (1.9-fold), and Bcl-2 (2.7-fold) were upregulated in the PHD1IR compared with WTIR, which was studied by real-time quantitative polymerase chain reaction. Further, gel-shift analysis showed increased DNA binding activity of HIF-1α and nuclear factor-kappaB in PHD1IR compared to WTIR. In addition, nuclear translocation of β-catenin was increased in PHD1IR compared with WTIR. These findings indicated that silencing of PHD-1 attenuates myocardial I/R injury probably by enhancing HIF-1α/β-catenin/endothelial nitric oxide synthase/nuclear factor-kappaB and Bcl-2 signaling pathway.
Figures
FIG. 1.
PHD-1 knockout does not appear to affect cardiac morphology and functions (echocardiographic evaluation) at baseline. (A) The validation of PHD-1−/− mice. The position of real-time polymerase chain reaction primers for PHD-1 mRNA is shown. Note that the exon 3-encoded region is deleted in PHD-1−/− mRNA. (B) Real-time polymerase chain reaction was performed using RNA isolated from myocardial tissue in WT and PHD-1−/− mice. The bands in PHD-1−/− mice were 120 bp shorter than WT bands. (C) Representative echocardiograph pictures of parasternal short-axis M mode images at baseline from WT and PHD-1−/− mice. (D) The quantitative data of systolic and diastolic LVID, and LVAW and LVPW thickness, and ejection fraction, fractional shortening, and heart rate from five different animals in each group. These results demonstrate that there was no difference in dynamic cardiac morphology and cardiac performance in the PHD-1−/− mice compared with WT mice. PHD-1−/− indicates PHD-1 knockout mice; HR, heart rate; bpm, beats per minute; LVIDs, left ventricular internal diameter in systole; LVIDd, LVID in diastole; LVPWs, left ventricular posterior wall thickness in systole; LVPWd, LVPW in diastole; LVAWd, left ventricular anterior wall thickness in diastole; LVAWs, LVAW in systole; EF, ejection fraction; FS, fractional shortening; WT, wild type.
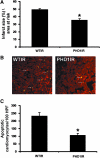
FIG. 2.
PHD-1−/− mice showed decreased infarct size and cardiomyocyte apoptosis after I/R. (A) The quantitative analysis from 6 different animals (n = 6) showed decreased infarct size in PHD1IR when compared to WTIR. (B) Representative digital micrographs showing cardiomyocyte apoptosis of PHD1IR and WTIR groups. (C) Quantitative analysis of cardiomyocyte apoptosis after I/R injury from 4 different animals in each group, in counts/100 high-power field (HPF). The apoptotic cardiomyocytes were significantly reduced in PHD1IR compared to WTIR. WTIR indicates WT animals subjected to I/R injury; PHD1IR, PHD-1−/− animals subjected to I/R injury. *p ≤ 0.05, versus WTIR. I/R, ischemia/reperfusion. (To see this illustration in color the reader is referred to the web version of this article at
).
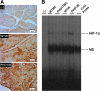
FIG. 3.
Effect of PHD-1 knockout on protein expression and DNA binding activity of HIF-1α after I/R. (A) Representative micrographs shows the protein expression of HIF-1α (mol. wt. ∼115 kDa) after I/R from different groups. The experiments were performed from three to four animals of each group. (B) EMSA analysis of DNA binding activity of HIF-1α (n = 4 from each group). These results showed increased expression, nuclear translocation, and DNA binding activity of HIF-1α in PHD1IR compared with WTIR. In EMSA, the position of the HIF-1α band was confirmed by the disappearance of the corresponding band in the last lane from right side when we used unlabelled probe (cold probe). WTMC indicates WT animals' time-matched control; PHD1TMC, PHD-1−/− animals time-matched control. HIF-1α, hypoxia-inducible transcription factor-1α; EMSA, electrophoretic mobility shift assay. (To see this illustration in color the reader is referred to the web version of this article at
).
FIG. 4.
Effect of PHD-1 knockout on mRNA expression of β-catenin, eNOS and Bcl-2, and nuclear translocation of β-catenin after I/R. Bar graphs show the mRNA expression of eNOS (A), Bcl-2 (B), β-catenin (C), and after I/R from different groups. The values are mean ± SEM of 4–5 animals from each group. The results showed increased mRNA expression of eNOS, β-catenin, and Bcl-2 in PHD1IR compared to WTIR. (D) Representative digital micrographs (immunohistochemical analysis by DAB staining and counter nuclear staining by hematoxylin) showing β-catenin (mol. wt. ∼92 kDa) nuclear translocation after I/R in different experimental groups; n = 4–5 per group. There was a significant increase in the expression of β-catenin as well as translocation of β-catenin into the nucleus in PHD1IR compared to WTIR. The value on top of the bars represents the two-tailed _p_-value (statistical difference) between WTIR and PHD1IR. eNOS, endothelial nitric oxide synthase. (To see this illustration in color the reader is referred to the web version of this article at
).
FIG. 5.
Effect of PHD-1 knockout on expression of p65 (subunit of NF-κB) and DNA binding activity of NF-κB after I/R. (A) Bar graph shows the mRNA expression of p65 in different groups after I/R injury. The values are mean ± SEM of 3–4 animals from each group. (B) EMSA analysis of DNA binding activity of NF-κB (n = 4 from each group). These results showed increased expression, nuclear translocation, and DNA binding activity of NF-κB in PHD1IR compared to WTIR. The position of the NF-κB band was confirmed by the super shift band with NF-κB antibody. The super shift band is given in separate box, which was longer exposure of the same blot of NF-κB. The value on top of the bars represents the two-tailed _p_-value (statistical difference) between WTIR and PHD1IR. NF-κB, nuclear factor-kappaB.
Similar articles
- Depletion of PHD3 protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis.
Xie L, Pi X, Wang Z, He J, Willis MS, Patterson C. Xie L, et al. J Mol Cell Cardiol. 2015 Mar;80:156-65. doi: 10.1016/j.yjmcc.2015.01.007. Epub 2015 Jan 26. J Mol Cell Cardiol. 2015. PMID: 25633836 Free PMC article. - Hypoxia inducible factor-1 activation by prolyl 4-hydroxylase-2 gene silencing attenuates myocardial ischemia reperfusion injury.
Natarajan R, Salloum FN, Fisher BJ, Kukreja RC, Fowler AA 3rd. Natarajan R, et al. Circ Res. 2006 Jan 6;98(1):133-40. doi: 10.1161/01.RES.0000197816.63513.27. Epub 2005 Nov 23. Circ Res. 2006. PMID: 16306444 - HIF-prolyl hydroxylases and cardiovascular diseases.
Sen Banerjee S, Thirunavukkarasu M, Tipu Rishi M, Sanchez JA, Maulik N, Maulik G. Sen Banerjee S, et al. Toxicol Mech Methods. 2012 Jun;22(5):347-58. doi: 10.3109/15376516.2012.673088. Toxicol Mech Methods. 2012. PMID: 22424133 Review. - Activation of hypoxia response in endothelial cells contributes to ischemic cardioprotection.
Kerkelä R, Karsikas S, Szabo Z, Serpi R, Magga J, Gao E, Alitalo K, Anisimov A, Sormunen R, Pietilä I, Vainio L, Koch WJ, Kivirikko KI, Myllyharju J, Koivunen P. Kerkelä R, et al. Mol Cell Biol. 2013 Aug;33(16):3321-9. doi: 10.1128/MCB.00432-13. Epub 2013 Jun 17. Mol Cell Biol. 2013. PMID: 23775121 Free PMC article. - Hypoxia-inducible factor as a therapeutic target for cardioprotection.
Ong SG, Hausenloy DJ. Ong SG, et al. Pharmacol Ther. 2012 Oct;136(1):69-81. doi: 10.1016/j.pharmthera.2012.07.005. Epub 2012 Jul 16. Pharmacol Ther. 2012. PMID: 22800800 Review.
Cited by
- Hypoxia-Inducible Factor and Its Role in the Management of Anemia in Chronic Kidney Disease.
Kaplan JM, Sharma N, Dikdan S. Kaplan JM, et al. Int J Mol Sci. 2018 Jan 29;19(2):389. doi: 10.3390/ijms19020389. Int J Mol Sci. 2018. PMID: 29382128 Free PMC article. Review. - LDL suppresses angiogenesis through disruption of the HIF pathway via NF-κB inhibition which is reversed by the proteasome inhibitor BSc2118.
Yao G, Zhang Q, Doeppner TR, Niu F, Li Q, Yang Y, Kuckelkorn U, Hagemann N, Li W, Hermann DM, Dai Y, Zhou W, Jin F. Yao G, et al. Oncotarget. 2015 Oct 6;6(30):30251-62. doi: 10.18632/oncotarget.4943. Oncotarget. 2015. PMID: 26388611 Free PMC article. - Circadian-Hypoxia Link and its Potential for Treatment of Cardiovascular Disease.
Bartman CM, Eckle T. Bartman CM, et al. Curr Pharm Des. 2019;25(10):1075-1090. doi: 10.2174/1381612825666190516081612. Curr Pharm Des. 2019. PMID: 31096895 Free PMC article. Review. - Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor-1α and upregulation of peroxisome proliferator-activated receptor-α.
Matsushima S, Kuroda J, Ago T, Zhai P, Ikeda Y, Oka S, Fong GH, Tian R, Sadoshima J. Matsushima S, et al. Circ Res. 2013 Apr 12;112(8):1135-49. doi: 10.1161/CIRCRESAHA.111.300171. Epub 2013 Mar 8. Circ Res. 2013. PMID: 23476056 Free PMC article. - The BMAL1/HIF2A heterodimer modulates circadian variations of myocardial injury.
Ruan W, Li T, Lee J, Bang IH, Deng W, Ma X, Yoo SH, Kim B, Li J, Yuan X, An YA, Wang YY, Liang Y, Deberge M, Zhang D, Zhou Z, Wang Y, Gorham J, Seidman JG, Seidman CE, Aranki SF, Nair R, Li L, Narula J, Zhao Z, Abebe AG, Muehlschlegel JD, Tsai KL, Eltzschig HK. Ruan W, et al. Res Sq [Preprint]. 2024 Feb 28:rs.3.rs-3938716. doi: 10.21203/rs.3.rs-3938716/v1. Res Sq. 2024. PMID: 38464103 Free PMC article. Updated. Preprint.
References
- Aragones J. Schneider M. Van Geyte K. Fraisl P. Dresselaers T. Mazzone M. Dirkx R. Zacchigna S. Lemieux H. Jeoung NH. Lambrechts D. Bishop T. Lafuste P. Diez-Juan A. Harten SK. Van Noten P. De Bock K. Willam C. Tjwa M. Grosfeld A. Navet R. Moons L. Vandendriessche T. Deroose C. Wijeyekoon B. Nuyts J. Jordan B. Silasi-Mansat R. Lupu F. Dewerchin M. Pugh C. Salmon P. Mortelmans L. Gallez B. Gorus F. Buyse J. Sluse F. Harris RA. Gnaiger E. Hespel P. Van Hecke P. Schuit F. Van Veldhoven P. Ratcliffe P. Baes M. Maxwell P. Carmeliet P. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. - PubMed
- Bao W. Qin P. Needle S. Erickson-Miller CL. Duffy KJ. Ariazi JL. Zhao S. Olzinski AR. Behm DJ. Pipes GC. Jucker BM. Hu E. Lepore JJ. Willette RN. Chronic inhibition of hypoxia-inducible factor prolyl 4-hydroxylase improves ventricular performance, remodeling, and vascularity after myocardial infarction in the rat. J Cardiovasc Pharmacol. 2010;56:147–155. - PubMed
- Eckle T. Kohler D. Lehmann R. El Kasmi K. Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. - PubMed
- Foadoddini M. Esmailidehaj M. Mehrani H. Sadraei SH. Golmanesh L. Wahhabaghai H. Valen G. Khoshbaten A. Pretreatment with hyperoxia reduces in vivo infarct size and cell death by apoptosis with an early and delayed phase of protection. Eur J Cardiothorac Surg. 2011;39:233–240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases