Exploring the P-glycoprotein binding cavity with polyoxyethylene alkyl ethers - PubMed (original) (raw)
Exploring the P-glycoprotein binding cavity with polyoxyethylene alkyl ethers
Xiaochun Li-Blatter et al. Biophys J. 2010.
Abstract
P-glycoprotein (ABCB1) moves allocrits from the cytosolic to the extracellular membrane leaflet, preventing their intrusion into the cytosol. It is generally accepted that allocrit binding from water to the cavity lined by the transmembrane domains occurs in two steps, a lipid-water partitioning step, and a cavity-binding step in the lipid membrane, whereby hydrogen-bond (i.e., weak electrostatic) interactions play a crucial role. The remaining key question was whether hydrophobic interactions also play a role for allocrit binding to the cavity. To answer this question, we chose polyoxyethylene alkyl ethers, C(m)EO(n), varying in the number of methylene and ethoxyl residues as model allocrits. Using isothermal titration calorimetry, we showed that the lipid-water partitioning step was purely hydrophobic, increasing linearly with the number of methylene, and decreasing with the number of ethoxyl residues, respectively. Using, in addition, ATPase activity measurements, we demonstrated that allocrit binding to the cavity required minimally two ethoxyl residues and increased linearly with the number of ethoxyl residues. The analysis provides the first direct evidence, to our knowledge, that allocrit binding to the cavity is purely electrostatic, apparently without any hydrophobic contribution. While the polar part of allocrits forms weak electrostatic interactions with the cavity, the hydrophobic part seems to remain associated with the lipid membrane. The interplay between the two types of interactions is most likely essential for allocrit flipping.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
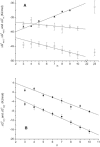
Figure 1
Free energies of binding of C12EOn (n = 3–10, 23) to membrane and transporter as a function of the number of ethoxyl groups (n) (T = 37°C). (A) Free energy of lipid-water partitioning, ΔGlw0 (▴); free energy of transporter-water binding (first binding region) ΔGtw(1)0 (□), free energy of transporter-water binding (second binding region) ΔGtw(2)0 (○), both derived from ATPase activity measurements. (B) Free energy of transporter-lipid binding (first binding region) ΔGtl(1)0 (■), (second binding region) ΔGtw(2)0 (●). Linear fits were performed in the range of n = 4–10. Values for ΔGtw(1)0 and ΔGtw(2)0 are the averages of at least five measurements.
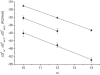
Figure 2
Free energies of binding of CmEO8 (m = 10, 12, 14) to the membrane and to the transporter as a function of the number of methylene groups (m). Free energy of lipid-water partitioning, ΔGlw0 (▴) (measured at T = 25°C and calculated at 37°C), free energy of transporter-water binding (first binding region) ΔGtw(1)0 (▪), and free energy of transporter-water binding (second binding region) ΔGtw(2)0 (●) (T = 37°C).
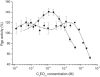
Figure 3
P-gp ATPase activity measured as a function of concentration of C12EOn (n = 3–10, 23) in plasma membrane vesicles of NIH-MDR1-G185 cells (T = 37°C and pH 7.0). C12EO3 (▪), C12EO4 (★), C12EO5 ( ), C12EO6 (▴), C12EO7 (
), C12EO6 (▴), C12EO7 ( ), C12EO8 (●), C12EO9 (♦), C12EO10 (◂), and C12EO23 (○). Data are expressed as the average of two measurements. (Solid lines) Fits to Eq. 4.
), C12EO8 (●), C12EO9 (♦), C12EO10 (◂), and C12EO23 (○). Data are expressed as the average of two measurements. (Solid lines) Fits to Eq. 4.
Figure 4
P-gp ATPase activity measured as a function of concentration of CmEO8 (m = 10, 12, 14) in plasma membrane vesicles of NIH-MDR1-G185 cells (T = 37°C and pH 7.0). C10EO8 (▪), C12EO8 (●), and C14EO8 (♦). Data are expressed as the average of two measurements. (Solid lines) Fits to Eq. 4.
Figure 5
Detergent mole fraction X(1) (▪) and X(2) (○) at the concentration of half-maximum activation, K(1), and inhibition, K(2), as a function of the number of ethoxyl groups, n, at 37°C.
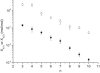
Figure 6
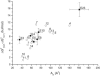
Difference in the free energy of binding from water to the activating and inhibitory binding region of P-gp, ΔGtw(2)0 – ΔGtw(1)0, as a function of the cross-sectional area, _A_D, of allocrits, determined by surface activity measurements. Drugs measured previously (Fig. 6 in (16)) (○). E(n) corresponds to C12EOn (n = 3, 4, 5, 8, 10, 23) (■).
Similar articles
- P-glycoprotein-ATPase modulation: the molecular mechanisms.
Li-Blatter X, Beck A, Seelig A. Li-Blatter X, et al. Biophys J. 2012 Mar 21;102(6):1383-93. doi: 10.1016/j.bpj.2012.02.018. Epub 2012 Mar 20. Biophys J. 2012. PMID: 22455921 Free PMC article. - Sav1866 from Staphylococcus aureus and P-glycoprotein: similarities and differences in ATPase activity assessed with detergents as allocrites.
Beck A, Aänismaa P, Li-Blatter X, Dawson R, Locher K, Seelig A. Beck A, et al. Biochemistry. 2013 May 14;52(19):3297-309. doi: 10.1021/bi400203d. Epub 2013 May 3. Biochemistry. 2013. PMID: 23600489 - Detergents as intrinsic P-glycoprotein substrates and inhibitors.
Li-Blatter X, Nervi P, Seelig A. Li-Blatter X, et al. Biochim Biophys Acta. 2009 Oct;1788(10):2335-44. doi: 10.1016/j.bbamem.2009.07.010. Epub 2009 Jul 22. Biochim Biophys Acta. 2009. PMID: 19631191 - P-glycoprotein (ABCB1) - weak dipolar interactions provide the key to understanding allocrite recognition, binding, and transport.
Seelig A, Li-Blatter X. Seelig A, et al. Cancer Drug Resist. 2023 Jan 1;6(1):1-29. doi: 10.20517/cdr.2022.59. eCollection 2023. Cancer Drug Resist. 2023. PMID: 37070101 Free PMC article. Review. - The P-glycoprotein multidrug transporter: interactions with membrane lipids, and their modulation of activity.
Sharom FJ. Sharom FJ. Biochem Soc Trans. 1997 Aug;25(3):1088-96. doi: 10.1042/bst0251088. Biochem Soc Trans. 1997. PMID: 9388605 Review. No abstract available.
Cited by
- Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function.
Sharom FJ. Sharom FJ. Front Oncol. 2014 Mar 3;4:41. doi: 10.3389/fonc.2014.00041. eCollection 2014. Front Oncol. 2014. PMID: 24624364 Free PMC article. Review. - Refined structures of mouse P-glycoprotein.
Li J, Jaimes KF, Aller SG. Li J, et al. Protein Sci. 2014 Jan;23(1):34-46. doi: 10.1002/pro.2387. Epub 2013 Nov 15. Protein Sci. 2014. PMID: 24155053 Free PMC article. - Effects of a detergent micelle environment on P-glycoprotein (ABCB1)-ligand interactions.
Shukla S, Abel B, Chufan EE, Ambudkar SV. Shukla S, et al. J Biol Chem. 2017 Apr 28;292(17):7066-7076. doi: 10.1074/jbc.M116.771634. Epub 2017 Mar 10. J Biol Chem. 2017. PMID: 28283574 Free PMC article. - Revealing the fate of cell surface human P-glycoprotein (ABCB1): The lysosomal degradation pathway.
Katayama K, Kapoor K, Ohnuma S, Patel A, Swaim W, Ambudkar IS, Ambudkar SV. Katayama K, et al. Biochim Biophys Acta. 2015 Oct;1853(10 Pt A):2361-70. doi: 10.1016/j.bbamcr.2015.06.001. Epub 2015 Jun 6. Biochim Biophys Acta. 2015. PMID: 26057472 Free PMC article. - Toward Novel [18F]Fluorine-Labeled Radiotracers for the Imaging of α-Synuclein Fibrils.
Uzuegbunam BC, Li J, Paslawski W, Weber W, Svenningsson P, Ågren H, Yousefi BH. Uzuegbunam BC, et al. Front Aging Neurosci. 2022 Apr 29;14:830704. doi: 10.3389/fnagi.2022.830704. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35572127 Free PMC article.
References
- Raviv Y., Pollard H.B., Gottesman M.M. Photosensitized labeling of a functional multidrug transporter in living drug-resistant tumor cells. J. Biol. Chem. 1990;265:3975–3980. - PubMed
- Shapiro A.B., Ling V. Extraction of Hoechst 33342 from the cytoplasmic leaflet of the plasma membrane by P-glycoprotein. Eur. J. Biochem. 1997;250:122–129. - PubMed
- Higgins C.F., Gottesman M.M. Is the multidrug transporter a flippase? Trends Biochem. Sci. 1992;17:18–21. - PubMed
- Nervi P., Li-Blatter X., Seelig A. P-glycoprotein substrate transport assessed by comparing cellular and vesicular ATPase activity. Biochim. Biophys. Acta. 2010;1798:515–525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources