Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4 - PubMed (original) (raw)
Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4
Bo Hu et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Chronic inflammation is a known risk factor for tumorigenesis, yet the precise mechanism of this association is currently unknown. The inflammasome, a multiprotein complex formed by NOD-like receptor (NLR) family members, has recently been shown to orchestrate multiple innate and adaptive immune responses, yet its potential role in inflammation-induced cancer has been little studied. Using the azoxymethane and dextran sodium sulfate colitis-associated colorectal cancer model, we show that caspase-1-deficient (Casp1(-/-)) mice have enhanced tumor formation. Surprisingly, the role of caspase-1 in tumorigenesis was not through regulation of colonic inflammation, but rather through regulation of colonic epithelial cell proliferation and apoptosis. Consequently, caspase-1-deficient mice demonstrate increased colonic epithelial cell proliferation in early stages of injury-induced tumor formation and reduced apoptosis in advanced tumors. We suggest a model in which the NLRC4 inflammasome is central to colonic inflammation-induced tumor formation through regulation of epithelial cell response to injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
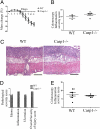
Caspase-1 deficiency exacerbates AOM-DSS–induced colon cancer. (A) Schematic overview of the inflammation-induced cancer model. (B) Tumor load and tumor numbers/mouse in WT and caspase-1 KO (Casp1−/−) mice (n = 8). P values < 0.05 were considered statistically significant. The experiments were repeated five times. (C) Representative colonoscopic appearance of WT and Casp1−/− mice colon on day 65 of AOM-DSS–induced color cancer. (D) Representative histopathologic sections of colon adenocarcinomas (I) and foci of tumor invasion (II) from WT and Casp1−/− mice. Invasive tumor foci (arrows) were smaller and less frequent in WT mice than Casp1−/− mice, where tumor foci were surrounded by abundant amounts of pale blue mucin. H&E staining; *muscularis mucosae; m, mascularis externa. (Scale bars, 200 μm.)
Fig. 2.
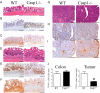
No differences in inflammation between WT and Casp1−/− mice during acute DSS colitis. There were no significant difference in percent-mass change (A), colonoscopy inflammation severity score (B), severity of histopathological morphology (C) (H&E staining), or histopathology parameters for edema, inflammation, ulceration or overall severity of injury (D) between WT and Casp1−/− mice. (Scale bars in C, 200 μm.) (E) Likewise, the severity of chronic DSS colitis was similar between WT and Casp1−/− mice. All experiments were repeated twice.
Fig. 3.
Enhanced colon epithelial and tumor cell proliferation in Casp1−/− mice during inflammation-induced colorectal cancer. Representative H&E, Ki67, and BrdU immunohistochemistry at day 15 (A–F) and at day 65 (G–I), and TUNEL-positive cells in colons (day 8) and tumors (day 65) in WT and Casp1−/− mice given AOM-DSS. The majority of crypts at day 15 appeared normal (A); however, there were increased Ki67+ (B) and BrdU+ (C) crypt epithelial cells in Casp1−/− mice compared with WT mice. In foci of crypt hyperplasia/dysplasia (D), there were no significant difference in the number of Ki6+ cells (E) and a moderate increase in the number of BrdU+ cells (F) in Casp1−/−compared with WT mice. At day 65 there were numerous large colon adenocarcinomas (G) with increased numbers of Ki67+ tumor cells (H) and BrdU+ tumor cells (I) in Casp1−/− compared with WT mice. (A, D, G: H&E staining; B, C, E, F, H, and I: DAB, Hematoxalyn) (Scale bars, 200 μm). (J) The number of TUNEL-positive cells in colon is similar in WT compared with Casp1−/− mice. In contrast, there is reduced cell death of tumor tissue in Casp1−/− mice (average cells counted: WT: 34.27 ± 8.964 positive cells/293.6 ± 86.39 total cells per field; Casp1−/−: 16.87 ± 4.533 positive cells/364.2 ± 98.70 total cells per field). DNA fragmentation in WT and Casp-1−/− mice was determined on either whole-colon sections on day 8 of AOM-DSS model or tumor tissues at day 65. Error bars represent ± SEM, P < 0.001. The experiments were repeated two to three times.
Fig. 4.
Caspase-1–mediated tumor enhancement is mediated by the NLRC4 inflammasome. (A) Caspase-1, NLRP3, and NLRC4 mRNA expression in colon epithelial cells and CD45+ cells. The levels of the indicated mRNAs were quantitated by real-time PCR and normalized to the level of HPRT mRNA. (B) Inflammation–induced tumor formation is similar in WT and NLRP3−/− mice (n ≥ 10). The experiments were repeated three times. (C) NLRC4−/− mice feature enhanced inflammation-induced colon cancer compared with WT mice (n ≥ 6); P < 0.05 was considered statistically significant. The experiments were repeated twice. Severity of DSS colitis in WT and NLRC4−/− mice, as evident by (D) mass change and (E) inflammation colonoscopy severity score. The experiments were repeated twice. (F) Caspase-1 mRNA expression in normal and colon and adjacent tumors from WT mice. mRNA levels were assessed by real-time PCR and normalized to the level of HPRT (n ≥ 5). The experiment was repeated twice. P < 0.05 was considered statistically significant. (G) Caspase-1 mRNA expression in naive and AOM-DSS treated proximal and distal colonic epithelial cells from WT mice. mRNA levels were assessed by real-time PCR and normalized to the level of HPRT (n ≥ 8). The experiment was repeated twice. P < 0.05 was considered statistically significant.
Similar articles
- Human Colon Tumors Express a Dominant-Negative Form of SIGIRR That Promotes Inflammation and Colitis-Associated Colon Cancer in Mice.
Zhao J, Bulek K, Gulen MF, Zepp JA, Karagkounis G, Martin BN, Zhou H, Yu M, Liu X, Huang E, Fox PL, Kalady MF, Markowitz SD, Li X. Zhao J, et al. Gastroenterology. 2015 Dec;149(7):1860-1871.e8. doi: 10.1053/j.gastro.2015.08.051. Epub 2015 Sep 5. Gastroenterology. 2015. PMID: 26344057 Free PMC article. - A Novel Role of Spred2 in the Colonic Epithelial Cell Homeostasis and Inflammation.
Takahashi S, Yoshimura T, Ohkura T, Fujisawa M, Fushimi S, Ito T, Itakura J, Hiraoka S, Okada H, Yamamoto K, Matsukawa A. Takahashi S, et al. Sci Rep. 2016 Nov 21;6:37531. doi: 10.1038/srep37531. Sci Rep. 2016. PMID: 27869219 Free PMC article. - Epimorphin deletion protects mice from inflammation-induced colon carcinogenesis and alters stem cell niche myofibroblast secretion.
Shaker A, Swietlicki EA, Wang L, Jiang S, Onal B, Bala S, DeSchryver K, Newberry R, Levin MS, Rubin DC. Shaker A, et al. J Clin Invest. 2010 Jun;120(6):2081-93. doi: 10.1172/JCI40676. Epub 2010 May 10. J Clin Invest. 2010. PMID: 20458144 Free PMC article. - Advances in Understanding Activation and Function of the NLRC4 Inflammasome.
Sundaram B, Kanneganti TD. Sundaram B, et al. Int J Mol Sci. 2021 Jan 21;22(3):1048. doi: 10.3390/ijms22031048. Int J Mol Sci. 2021. PMID: 33494299 Free PMC article. Review. - Inflammasome-mediated suppression of inflammation-induced colorectal cancer progression is mediated by direct regulation of epithelial cell proliferation.
Hu B, Elinav E, Flavell RA. Hu B, et al. Cell Cycle. 2011 Jun 15;10(12):1936-9. doi: 10.4161/cc.10.12.16008. Epub 2011 Jun 15. Cell Cycle. 2011. PMID: 21555913 Review.
Cited by
- Tissue-specific opposing functions of the inflammasome adaptor ASC in the regulation of epithelial skin carcinogenesis.
Drexler SK, Bonsignore L, Masin M, Tardivel A, Jackstadt R, Hermeking H, Schneider P, Gross O, Tschopp J, Yazdi AS. Drexler SK, et al. Proc Natl Acad Sci U S A. 2012 Nov 6;109(45):18384-9. doi: 10.1073/pnas.1209171109. Epub 2012 Oct 22. Proc Natl Acad Sci U S A. 2012. PMID: 23090995 Free PMC article. - Cancer and the microbiota.
Garrett WS. Garrett WS. Science. 2015 Apr 3;348(6230):80-6. doi: 10.1126/science.aaa4972. Science. 2015. PMID: 25838377 Free PMC article. Review. - Noncoding RNA-mediated regulation of pyroptotic cell death in cancer.
Wang M, Zhang Y, Chang W, Zhang L, Syrigos KN, Li P. Wang M, et al. Front Oncol. 2022 Oct 31;12:1015587. doi: 10.3389/fonc.2022.1015587. eCollection 2022. Front Oncol. 2022. PMID: 36387211 Free PMC article. Review. - Defective Intestinal Mucin-Type O-Glycosylation Causes Spontaneous Colitis-Associated Cancer in Mice.
Bergstrom K, Liu X, Zhao Y, Gao N, Wu Q, Song K, Cui Y, Li Y, McDaniel JM, McGee S, Chen W, Huycke MM, Houchen CW, Zenewicz LA, West CM, Chen H, Braun J, Fu J, Xia L. Bergstrom K, et al. Gastroenterology. 2016 Jul;151(1):152-164.e11. doi: 10.1053/j.gastro.2016.03.039. Epub 2016 Apr 6. Gastroenterology. 2016. PMID: 27059389 Free PMC article. - NLRP1 in Cutaneous SCCs: An Example of the Complex Roles of Inflammasomes in Cancer Development.
Di Filippo M, Hennig P, Karakaya T, Slaufova M, Beer HD. Di Filippo M, et al. Int J Mol Sci. 2022 Oct 14;23(20):12308. doi: 10.3390/ijms232012308. Int J Mol Sci. 2022. PMID: 36293159 Free PMC article. Review.
References
- Weir HK, et al. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95:1276–1299. - PubMed
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. - PubMed
- Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–183. - PubMed
- Askling J, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356–1362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases