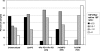Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines - PubMed (original) (raw)
Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines
Jan Poolman et al. Clin Vaccine Immunol. 2011 Feb.
Abstract
7vCRM (Pfizer, Inc.) and PHiD-CV (GlaxoSmithKline Biologicals) are two pneumococcal conjugate vaccines licensed for the prevention of invasive pneumococcal disease and acute otitis media caused by the vaccine serotypes of Streptococcus pneumoniae. Neither vaccine contains serotype 19A, but both contain the closely related serotype 19F. No decrease in the incidence of serotype 19A disease has been observed following the introduction of 7vCRM, suggesting that this serotype 19F-containing vaccine provides limited cross-protection against serotype 19A. To investigate the impact that conjugation methods may have on antipolysaccharide immune responses and to determine whether this limited cross-protection is characteristic of the serotype 19F polysaccharide or rather of the 19F-CRM (cross-reacting material) conjugate, we compared naturally induced antibodies against serotypes 19F and 19A with antibodies induced after vaccination with different pneumococcal conjugate vaccines. We found that conjugation of the serotype 19F polysaccharide using reductive amination (as in 7vCRM) resulted in the formation of at least one additional epitope that is not present in the native form of the 19F polysaccharide or following 19F conjugation using a bifunctional spacer (as in the prototype vaccine 7vOMPC) or cyanylation (as in PHiD-CV). We also found that pneumococcal vaccines conjugated using cyanylation induce more opsonophagocytic antibodies against serotype 19F and a considerably higher level of cross-opsonophagocytic antibodies against serotype 19A than vaccines conjugated using reductive amination. In conclusion, these results suggest that the conjugation method can influence the functionality of the antibodies induced against the homologous serotype 19F and the cross-reactive serotype 19A of S. pneumoniae.
Figures
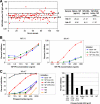
FIG. 1.
Antibody binding to 19F polysaccharide antigens. (A) Concentrations of IgG antibodies to native 19F polysaccharide and to the 19F polysaccharide conjugated to human serum albumin (19F-HSA) by reductive amination in sera from healthy unvaccinated blood donors (n = 113) were measured by 22F-ELISA as described previously (7). The 19F-HSA/native 19F IgG concentration ratio was calculated for each donor. (B) The inhibition of anti-19F polysaccharide (PS) binding was measured in two sera (one [NA-31] with similar concentrations of antibodies to native 19F and 19F-HSA and the other [NA-47] with a high concentration of the antibody to 19F-HSA) from healthy unimmunized adults by inhibition ELISA, as described previously (7). ELISA plates were coated with either native 19F polysaccharide, the 19F polysaccharide linked to HSA (19F-HSA) by reductive amination (RA), or the 19F polysaccharide linked to tyramine (19F-tyra) by cyanylation (CN). (C) The binding of different 19F conjugates and polysaccharides from the related serotypes 19A, 19B, and 19C was measured in serum NA-47 as for panel B.
FIG. 2.
Comparative distribution of ratios of the concentration of IgG against 19F-HSA to the concentration of IgG against native 19F polysaccharide. The 19F-HSA/native 19F IgG antibody concentration ratio was calculated for sera from healthy unimmunized adults (n = 113) or from adults vaccinated with either 23vPS (n = 24), 7vCRM (a vaccine conjugated using reductive amination [RA]) (n = 15), 7vOMPC (a prototype vaccine conjugated using a bigeneric spacer [BS]) (n = 33), or one of the two experimental vaccines 11Pn-PD and 4Pn-PD (both vaccines conjugated using cyanylation [CN]) (n = 19). The 22F-ELISA was performed as described previously (7).
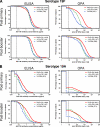
FIG. 3.
Reverse cumulative distribution curves of antipolysaccharide IgG concentrations and OPA titers measured in pediatric sera after vaccination with 7vCRM, PHiD-CV, or 11Pn-PD. (A) Serotype 19F; (B) serotype 19A. To prepare the reverse cumulative distribution curves for primary vaccination, the data from the PHiD-CV groups in studies A to C were pooled; the data from the 7vCRM groups in studies A to C were pooled; and the data from the POET study group (study G) were used for 11Pn-PD. For the booster vaccination, the data from the PHiD-CV groups in studies D to F were pooled; the data from the 7vCRM groups in studies D to F were pooled; and the data from the POET study group (study G) were used for 11Pn-PD. The dotted lines represent an assay cutoff of 0.05 μg/ml for the 22F-ELISA and a titer of 8 for the OPA assay. The numbers of sera tested are given in parentheses in the keys.
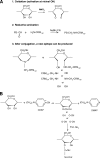
FIG. 4.
Preparation of polysaccharide-protein conjugates using reductive amination or cyanylation. (A) Conjugation involves oxidation of the polysaccharide with sodium periodate to introduce reactive aldehydes, followed by linkage to the carrier protein using reductive amination. This method breaks and opens the hexasaccharide ring structure. After conjugation, a new immunogenic epitope can be produced due to the binding of new groups to the hexasaccharide ring. (B) Cyanylation using CDAP introduces a cyanate group to hydroxyl groups, which forms a covalent bond to the amino or hydrazide group upon addition of the protein component. After cyanylation conjugation, the hexasaccharide ring remains intact and other chemical groups are not able to bind.
Similar articles
- Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A?
Hausdorff WP, Hoet B, Schuerman L. Hausdorff WP, et al. BMC Pediatr. 2010 Feb 2;10:4. doi: 10.1186/1471-2431-10-4. BMC Pediatr. 2010. PMID: 20122261 Free PMC article. Review. - Immunogenicity and safety of a novel ten-valent pneumococcal conjugate vaccine in healthy infants in The Gambia: a phase 3, randomised, double-blind, non-inferiority trial.
Clarke E, Bashorun A, Adigweme I, Badjie Hydara M, Umesi A, Futa A, Ochoge M, Obayemi D, Edem B, Saidy-Jah E, Onwuchekwa C, Dhere R, Sethna V, Kampmann B, Goldblatt D, Taylor D, Andi-Lolo I, Hosken N, Antony K, Innis BL, Alderson MR, Lamola S. Clarke E, et al. Lancet Infect Dis. 2021 Jun;21(6):834-846. doi: 10.1016/S1473-3099(20)30735-0. Epub 2021 Jan 28. Lancet Infect Dis. 2021. PMID: 33516293 Clinical Trial. - Immunogenicity and safety of 11- and 12-valent pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccines (11vPHiD-CV, 12vPHiD-CV) in infants: Results from a phase II, randomised, multicentre study.
Carmona Martinez A, Prymula R, Miranda Valdivieso M, Otero Reigada MDC, Merino Arribas JM, Brzostek J, Szenborn L, Ruzkova R, Horn MR, Jackowska T, Centeno-Malfaz F, Traskine M, Dobbelaere K, Borys D. Carmona Martinez A, et al. Vaccine. 2019 Jan 3;37(1):176-186. doi: 10.1016/j.vaccine.2018.07.023. Epub 2018 Jul 24. Vaccine. 2019. PMID: 30054160 Clinical Trial. - Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine.
Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsène JP, Lommel P, Dieussaert I, Schuerman L. Vesikari T, et al. Pediatr Infect Dis J. 2009 Apr;28(4 Suppl):S66-76. doi: 10.1097/INF.0b013e318199f8ef. Pediatr Infect Dis J. 2009. PMID: 19325449 Clinical Trial. - Pneumococcal polysaccharide protein D-conjugate vaccine (Synflorix; PHiD-CV).
Croxtall JD, Keating GM. Croxtall JD, et al. Paediatr Drugs. 2009;11(5):349-57. doi: 10.2165/11202760-000000000-00000. Paediatr Drugs. 2009. PMID: 19725600 Review.
Cited by
- Draft Genome Sequences of Two Streptococcus pneumoniae Serotype 19F Sequence Type 271 Clinical Isolates with Low- and High-Level Cefotaxime Resistance.
Ip M, Ma H, Li C, Tsui S, Zhou H. Ip M, et al. Genome Announc. 2015 Jun 11;3(3):e00605-15. doi: 10.1128/genomeA.00605-15. Genome Announc. 2015. PMID: 26067956 Free PMC article. - Pneumococcal Capsules and Their Types: Past, Present, and Future.
Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. Geno KA, et al. Clin Microbiol Rev. 2015 Jul;28(3):871-99. doi: 10.1128/CMR.00024-15. Clin Microbiol Rev. 2015. PMID: 26085553 Free PMC article. Review. - Pneumococcal disease and use of pneumococcal vaccines in Taiwan.
Wei SH, Chiang CS, Chen CL, Chiu CH. Wei SH, et al. Clin Exp Vaccine Res. 2015 Jul;4(2):121-9. doi: 10.7774/cevr.2015.4.2.121. Epub 2015 Jul 29. Clin Exp Vaccine Res. 2015. PMID: 26273570 Free PMC article. Review. - Trends in therapeutic drug conjugates for bacterial diseases: a patent review.
Cal PM, Matos MJ, Bernardes GJ. Cal PM, et al. Expert Opin Ther Pat. 2017 Feb;27(2):179-189. doi: 10.1080/13543776.2017.1259411. Epub 2016 Nov 23. Expert Opin Ther Pat. 2017. PMID: 27828733 Free PMC article. Review. - Discovery of Semi- and Fully-Synthetic Carbohydrate Vaccines Against Bacterial Infections Using a Medicinal Chemistry Approach.
Seeberger PH. Seeberger PH. Chem Rev. 2021 Apr 14;121(7):3598-3626. doi: 10.1021/acs.chemrev.0c01210. Epub 2021 Apr 1. Chem Rev. 2021. PMID: 33794090 Free PMC article. Review.
References
- Bermal, N., et al. 2011. Safety and immunogenicity of a booster dose of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) coadministered with DTPw-HBV/Hib and poliovirus vaccines. Pediatr. Infect. Dis. J. 30:69-72. - PubMed
- Bermal, N., et al. 2009. The 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) co-administered with DTPw-HBV/Hib and poliovirus vaccines: assessment of immunogenicity. Pediatr. Infect. Dis. J. 28:S89-S96. - PubMed
- Black, S., et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 19:187-195. - PubMed
- Centers for Disease Control and Prevention. 2010. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb. Mortal. Wkly. Rep. 59:258-261. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources