Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells - PubMed (original) (raw)
Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells
Qinghe Chen et al. PLoS One. 2010.
Abstract
Background: Resveratrol, a naturally occurring phytopolyphenol compound, has attracted extensive interest in recent years because of its diverse pharmacological characteristics. Although resveratrol possesses chemopreventive properties against several cancers, the molecular mechanisms by which it inhibits cell growth and induces apoptosis have not been clearly understood. The present study was carried out to examine whether PI3K/AKT/FOXO pathway mediates the biological effects of resveratrol.
Methodology/principal findings: Resveratrol inhibited the phosphorylation of PI3K, AKT and mTOR. Resveratrol, PI3K inhibitors (LY294002 and Wortmannin) and AKT inhibitor alone slightly induced apoptosis in LNCaP cells. These inhibitors further enhanced the apoptosis-inducing potential of resveratrol. Overexpression of wild-type PTEN slightly induced apoptosis. Wild type PTEN and PTEN-G129E enhanced resveratrol-induced apoptosis, whereas PTEN-G129R had no effect on proapoptotic effects of resveratrol. Furthermore, apoptosis-inducing potential of resveratrol was enhanced by dominant negative AKT, and inhibited by wild-type AKT and constitutively active AKT. Resveratrol has no effect on the expression of FKHR, FKHRL1 and AFX genes. The inhibition of FOXO phosphorylation by resveratrol resulted in its nuclear translocation, DNA binding and transcriptional activity. The inhibition of PI3K/AKT pathway induced FOXO transcriptional activity resulting in induction of Bim, TRAIL, p27/KIP1, DR4 and DR5, and inhibition of cyclin D1. Similarly, resveratrol-induced FOXO transcriptional activity was further enhanced when activation of PI3K/AKT pathway was blocked. Over-expression of phosphorylation deficient mutants of FOXO proteins (FOXO1-TM, FOXO3A-TM and FOXO4-TM) induced FOXO transcriptional activity, which was further enhanced by resveratrol. Inhibition of FOXO transcription factors by shRNA blocked resveratrol-induced upregulation of Bim, TRAIL, DR4, DR5, p27/KIP1 and apoptosis, and inhibition of cyclin D1 by resveratrol.
Conclusion/significance: These data suggest that FOXO transcription factors mediate anti-proliferative and pro-apoptotic effects of resveratrol, in part due to activation of extrinsic apoptosis pathway.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
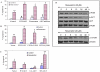
Figure 1. Inhibition of PI3K/AKT pathway enhances apoptosis-inducing potential of resveratrol.
(A), LNCaP cells were pretreated with LY294002 (1 µM), Wortmannin (1 µM) or AKT inhibitor (100 nM) for 2 h, followed by treatment with resveratrol (20 µM) for 48 h. At the end of incubation period, cells were harvested and apoptosis was measured by TUNEL assay. Data represent mean ± SE. *, # and ** = significantly different from respective control (P<0.05). (B), LNCaP cells were transiently transfected with empty vector, PTEN-WT, PTEN-G129E or PTEN-G129R. The culture medium was changed and cells were treated with or without resveratrol (20 µM) for 48 h, and apoptosis was measured by TUNEL assay. Data represent mean ± SE. *, **, % and $ = significantly different from respective control (P<0.05). (C), LNCaP cells were transiently transfected with empty vector, WT-AKT, CA-AKT or DN-AKT. The culture medium was changed and cells were treated with or without resveratrol (20 µM) for 48 h, and apoptosis was measured by TUNEL assay. Data represent mean ± SE. *, **, # and % = significantly different from respective control (P<0.05). (D), Effects of resveratrol on the expression of PI3K, AKT and mTOR pathway. LNCaP cells were treated with resveratrol (20 µM) for various time points. Western blots were performed with anti-phospho-PI3K, phospho-AKT, anti-AKT and phospho-mTOR antibodies. β-Actin was used as a loading control. The data are representative of three experiments.
Figure 2. Regulation of FOXO by resveratrol.
(A), Effects of resveratrol on the expression of FKHR, FKHRL1 and AFX. LNCaP cells were treated with resveratrol (10 or 20 µM) for 6, 12 or 24 h. Total RNA was isolated and RT-PCR analysis was performed to measure the expression of FKHR, FKHRL1 and AFX. (B), Effects of resveratrol on the phosphorylation of FOXO proteins. LNCaP cells were treated with resveratrol (20 µM) for 0–48 h, and the Western blot analyses were performed to measure the expression of phospho-FKHR, phospho-FKHRL1 and phosphor-AFX. β-Actin was used as a loading control. (C), Resveratrol induces translocation of FKHR to nucleus. LNCaP cells were seeded in chambered slides and transfected with GFP-FKHR or GFP-FKHR-TM (phosphorylation deficient triple mutant). The culture medium was changed and cells were treated with or without resveratrol (20 µM) for 24 h. Cells were fixed, permeabilized, and stained with DAPI and visualized under a fluorescence microscope. green color = FKHR, red color = nucleus (for clarity the color of nucleus was changed from blue to red), yellow = colocalization of FKHR to nucleus. (D), Left panel, LNCaP cells were treated with resveratrol (20 µM) for various time points (0–24 h). Nuclear extracts were prepared and the gelshift experiment was performed as described in Materials and Methods. Lane 1 = probe only, lanes 2–9 = resveratrol treated samples, lane 10 = cold probe (50×), and lane 11 = cold SP1 probe. The data are representative of three experiments. Right panel, LNCaP cells were untreated or treated with resveratrol (20 µM) in the presence or absence of LY (1 µM) or AKT inhibitor (100 nM) for 24 h. Nuclear extracts were prepared and the gelshift experiment was performed as described in Materials and Methods. Lane 1 = probe only, lane 2 = cold probe (50×), lane 3 = cold SP1, lane 4 = control, lane 5 = resveratrol, lane 6 = LY, lane 7 = AKT inhibitor, lane 8 = resveratrol plus LY, lane 9 = resveratrol + AKT inhibitor. The data are representative of three experiments.
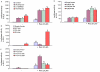
Figure 3. Inhibition of PI3K/AKT pathway or overexpression of phosphorylation deficient mutants of FOXO enhanced resveratrol-induced FOXO transcriptional activity.
(A), Pharmacological inhibition of PI3K/AKT pathway enhanced resveratrol-induced FOXO activity in LNCaP cells. LNCaP cells were transiently transfected with 6X DBE-luciferase and pRL-TK plasmids for 24 h as we described elsewhere. After transfection, cells were pretreated with Wortmannin (10 µM), LY-294002 (10 µM) or AKT inhibitor IV (1 µM) for 2 h, followed by treatment with or without resveratrol (20 µM) for 24 h. Cells were harvested for firefly/Renilla luciferase assays using the Dual-Luciferase Reporter Assay System (Promega). Luciferase counts were normalized using Renilla luciferase transfection control. Data represent the mean ± S.D. *, #, ** = significantly different from control, P<0.05. (B), Overexpression of dominant negative AKT enhanced resveratrol-induced FOXO activity. LNCaP cells were transiently transfected with 6X DBE-luciferase plus pRL-TK plasmids with WT-AKT, CA-AKT or DN-AKT for 24 h. After transfection, cells were pretreated with resveratrol (20 µM) for 24 h. Cells were harvested for firefly/Renilla luciferase assays using the Dual-Luciferase Reporter Assay System (Promega). Luciferase counts were normalized using Renilla luciferase transfection control. Data represent the mean ± S.D. *, # and ** = significantly different from control, P<0.05. (C), Regulation of resveratrol-induced FOXO activity by wild type or mutant PTEN. LNCaP cells were transiently transfected with 6X DBE-luciferase plus pRL-TK plasmids with PTEN-WT, PTEN-G129E or PTEN-G129R for 24 h. After transfection, cells were pretreated with resveratrol (20 µM) for 24 h. Cells were harvested for firefly/Renilla luciferase assays using the Dual-Luciferase Reporter Assay System (Promega). Luciferase counts were normalized using Renilla luciferase transfection control. Data represent the mean ± S.D. *, &, $ and ** = significantly different from control, P<0.05. (D), Phosphorylation deficient mutants of FOXO enhance resveratrol-induced FOXO transcriptional activity. LNCaP cells were transiently transfected with empty vector or constructs encoding FOXO1-TM, FOXO3a-TM, or FOXO4-TM together with 6X DBE-luciferase and pRL-TK plasmids for 24 h. After transfection, cells were washed, treated with resveratrol (20 µM) for 24 h, and harvested for firefly/Renilla luciferase assays using the Dual-Luciferase Reporter Assay System (Promega). Luciferase counts were normalized using Renilla luciferase transfection control. Data represent the mean ± S.D. * and ** = significantly different from control, P<0.05.
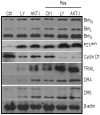
Figure 4. Effects of PI3K/AKT pathway and resveratrol on the expression of FOXO target genes.
LNCaP cells were pretreated with LY294002 (10 µM) or AKT inhibitor (AKT-I, 1 µM) for 2 h and treated with or without resveratrol (20 µM) for 48 h. Cells were harvested to measure the expression of Bim, p27/KIP1, cyclin D1, TRAIL, DR4 and DR5 by theWestern blot analysis. β-actin was used as a loading control.
Figure 5. Effects of FOXO transcription factors on the regulation of antiproliferative and proapoptotic effects of resveratrol.
(A), Inhibition of FOXO transcription factor by shRNA blocks anti-proliferative effects of resveratrol. LNCaP cells were transiently transfected with plasmids expressing FKHR shRNA, FKHRL1 shRNA, AFX shRNA or respective scrambled control and treated with resveratrol (20 µM) for 48 h, and cell viability was measured. (B), Inhibition of FOXO transcription factors or ROS (reactive oxygen species) by NAC blocks resveratrol-induced apoptosis. LNCaP cells were transfected with a mixture of plasmids expressing FKHR shRNA, FKHRL1 shRNA plus AFX shRNA or scrambled control. After transfection, the culture medium was changed and cells were pretreated with NAC for 2 h, and treated with resveratrol (20 µM) for 48 h. At the end of incubation period, the apoptosis was measured by TUNEL assay. (C), Inhibition of FOXO transcription factors or ROS by NAC blocks resveratrol-induced caspase-3 activity. LNCaP cells were transfected with a mixture of plasmids expressing FKHR shRNA, FKHRL1 shRNA plus AFX shRNA or scrambled control. After transfection, the culture medium was changed and cells were pretreated with NAC for 2 h, and treated with resveratrol (20 µM) for 48 h. At the end of incubation period, the caspase-3 activity was measured as per manufacturer instructions.
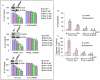
Figure 6. FOXO transcription factors mediate the expression of cell cycle and apoptosis-related genes.
(A), LNCaP cells were transfected with plasmids expressing FKHR shRNA or scrambled control. After transfection, the culture medium was changed and cells were treated with resveratrol (0–20 µM) for 48 h. At the end of incubation period, cells were harvested to measure the expression of Bim, TRAIL, DR4, DR5, p27 and cyclin D1 by the Western blot analysis. β-actin was used as a loading control. (B), LNCaP cells were transfected with plasmids expressing FKHRL1 shRNA or scrambled control. After transfection, the culture medium was changed and cells were treated with resveratrol (0–20 µM) for 48 h. At the end of incubation period, cells were harvested to measure the expression of Bim, TRAIL, DR4, DR5, p27 and cyclin D1 by the Western blot analysis. β-actin was used as a loading control. (C), LNCaP cells were transfected with plasmids expressing AFX shRNA or scrambled control. After transfection, the culture medium was changed and cells were treated with resveratrol (0–20 µM) for 48 h. At the end of incubation period, cells were harvested to measure the expression of Bim, TRAIL, DR4, DR5, p27 and cyclin D1 by the Western blot analysis. β-actin was used as a loading control.
Similar articles
- Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor.
Ganapathy S, Chen Q, Singh KP, Shankar S, Srivastava RK. Ganapathy S, et al. PLoS One. 2010 Dec 28;5(12):e15627. doi: 10.1371/journal.pone.0015627. PLoS One. 2010. PMID: 21209944 Free PMC article. - Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors.
Roy SK, Chen Q, Fu J, Shankar S, Srivastava RK. Roy SK, et al. PLoS One. 2011;6(9):e25166. doi: 10.1371/journal.pone.0025166. Epub 2011 Sep 27. PLoS One. 2011. PMID: 21980390 Free PMC article. - Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway.
Shukla S, Bhaskaran N, Babcook MA, Fu P, Maclennan GT, Gupta S. Shukla S, et al. Carcinogenesis. 2014 Feb;35(2):452-60. doi: 10.1093/carcin/bgt316. Epub 2013 Sep 25. Carcinogenesis. 2014. PMID: 24067903 Free PMC article. - Interplay Among PI3K/AKT, PTEN/FOXO and AR Signaling in Prostate Cancer.
Yan Y, Huang H. Yan Y, et al. Adv Exp Med Biol. 2019;1210:319-331. doi: 10.1007/978-3-030-32656-2_14. Adv Exp Med Biol. 2019. PMID: 31900915 Review. - FOXO transcription factors in cancer development and therapy.
Coomans de Brachène A, Demoulin JB. Coomans de Brachène A, et al. Cell Mol Life Sci. 2016 Mar;73(6):1159-72. doi: 10.1007/s00018-015-2112-y. Epub 2015 Dec 19. Cell Mol Life Sci. 2016. PMID: 26686861 Free PMC article. Review.
Cited by
- Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells.
Nassir AM, Shahzad N, Ibrahim IAA, Ahmad I, Md S, Ain MR. Nassir AM, et al. Saudi Pharm J. 2018 Sep;26(6):876-885. doi: 10.1016/j.jsps.2018.03.009. Epub 2018 Mar 15. Saudi Pharm J. 2018. PMID: 30202231 Free PMC article. - Resveratrol, Epigallocatechin Gallate and Curcumin for Cancer Therapy: Challenges from Their Pro-Apoptotic Properties.
Chimento A, D'Amico M, De Luca A, Conforti FL, Pezzi V, De Amicis F. Chimento A, et al. Life (Basel). 2023 Jan 17;13(2):261. doi: 10.3390/life13020261. Life (Basel). 2023. PMID: 36836619 Free PMC article. Review. - Resveratrol enhances antitumor activity of TRAIL in prostate cancer xenografts through activation of FOXO transcription factor.
Ganapathy S, Chen Q, Singh KP, Shankar S, Srivastava RK. Ganapathy S, et al. PLoS One. 2010 Dec 28;5(12):e15627. doi: 10.1371/journal.pone.0015627. PLoS One. 2010. PMID: 21209944 Free PMC article. - Ethnobiology and Ethnopharmacology of Lepidium meyenii (Maca), a Plant from the Peruvian Highlands.
Gonzales GF. Gonzales GF. Evid Based Complement Alternat Med. 2012;2012:193496. doi: 10.1155/2012/193496. Epub 2011 Oct 2. Evid Based Complement Alternat Med. 2012. PMID: 21977053 Free PMC article. - Crosstalk of the Wnt/β-Catenin Signaling Pathway in the Induction of Apoptosis on Cancer Cells.
Trejo-Solis C, Escamilla-Ramirez A, Jimenez-Farfan D, Castillo-Rodriguez RA, Flores-Najera A, Cruz-Salgado A. Trejo-Solis C, et al. Pharmaceuticals (Basel). 2021 Aug 28;14(9):871. doi: 10.3390/ph14090871. Pharmaceuticals (Basel). 2021. PMID: 34577571 Free PMC article. Review.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin 2010 - PubMed
- Shankar S, Siddiqui I, Srivastava RK. Molecular mechanisms of resveratrol (3,4,5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cell Biochem. 2007;304:273–285. - PubMed
- Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, et al. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 2010;70:3382–3390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous