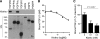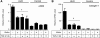Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice - PubMed (original) (raw)
. 2011 Mar 11;286(10):8655-8665.
doi: 10.1074/jbc.M110.174037. Epub 2011 Jan 5.
Yonglong Zou 2, Osamu Togao 3, Johanne V Pastor 1, George B John 1, Lei Wang 1, Kazuhiro Shiizaki 1, Russell Gotschall 4, Susan Schiavi 4, Noriaki Yorioka 5, Masaya Takahashi 3, David A Boothman 6, Makoto Kuro-O 7
Affiliations
- PMID: 21209102
- PMCID: PMC3048747
- DOI: 10.1074/jbc.M110.174037
Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice
Shigehiro Doi et al. J Biol Chem. 2011.
Abstract
Fibrosis is a pathological process characterized by infiltration and proliferation of mesenchymal cells in interstitial space. A substantial portion of these cells is derived from residing non-epithelial and/or epithelial cells that have acquired the ability to migrate and proliferate. The mesenchymal transition is also observed in cancer cells to confer the ability to metastasize. Here, we show that renal fibrosis induced by unilateral ureteral obstruction and metastasis of human cancer xenografts are suppressed by administration of secreted Klotho protein to mice. Klotho is a single-pass transmembrane protein expressed in renal tubular epithelial cells. The extracellular domain of Klotho is secreted by ectodomain shedding. Secreted Klotho protein directly binds to the type-II TGF-β receptor and inhibits TGF-β1 binding to cell surface receptors, thereby inhibiting TGF-β1 signaling. Klotho suppresses TGF-β1-induced epithelial-to-mesenchymal transition (EMT) responses in cultured cells, including decreased epithelial marker expression, increased mesenchymal marker expression, and/or increased cell migration. In addition to TGF-β1 signaling, secreted Klotho has been shown to inhibit Wnt and IGF-1 signaling that can promote EMT. These results have raised the possibility that secreted Klotho may function as an endogenous anti-EMT factor by inhibiting multiple growth factor signaling pathways simultaneously.
Figures
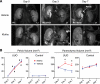
FIGURE 1.
Effects of Klotho protein injection on renal morphology in UUO mice determined by MRI. A, typical T2 weighted MR images from mice before (Day 0) and after UUO (Day 3 and Day 7) administered with vehicle (upper panels) or Klotho (0.02 mg/kg, lower panels) by intraperitoneal injection every 48 h. The arrow indicates enlarged pelvis. B, changes in the volume of pelvis and parenchyma determined by the MR images. Right kidneys (UUO) and left kidneys (Control) from Klotho-treated mice (red, n = 6) and vehicle-treated mice (blue, n = 4) were compared.
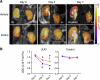
FIGURE 2.
Effects of Klotho protein injection on renal cell density in UUO mice determined by MRI. A, typical ADC maps from mice before (Day 0) and after UUO (Day 3 and Day 7) administered with vehicle (upper panels) or Klotho (0.02 mg/kg, lower panels). B, changes in the average ADC values. Data indicate means ± S.E. *, p < 0.05 versus vehicle-treated mice at the same time points by two-tailed t test. #, p < 0.05 versus mice at Day 0 by two-tailed t test.
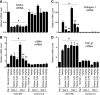
FIGURE 3.
Effects of Klotho protein injection on cell density and fibrosis in UUO mice determined by histological analysis. A, typical histology of UUO kidneys from mice treated with vehicle or Klotho. Paraffin sections of UUO kidneys were stained with hematoxylin-eosin staining. The arrow indicates infiltration of myofibroblasts in the interstitial space. B, quantification of cell density. Five fields on a coronal section were selected randomly, and the number of nuclei in each field was counted under a high power field (original magnification ×400) by using the ImageJ software as described previously (27). Data indicate means ± S.E. p < 0.01 between the Vehicle-treated group (n = 4) and the Klotho-treated group (n = 6) in UUO kidneys by two-tailed t test. C, typical histology of UUO kidneys from mice treated with vehicle or Klotho. Paraffin sections of UUO kidneys were stained with Masson's trichrome that detected connective tissue as blue staining. D, Quantification of fibrotic area. Five fields on the coronal section were randomly selected and the ratios of blue to total areas in each field were counted in a high power field (original magnification ×400) using the ImageJ software. Data indicate means ± S.E. p < 0.05 between the vehicle-treated group (n = 4) and the Klotho-treated group (n = 6) in UUO kidneys by two-tailed t test.
FIGURE 4.
Klotho protein injection suppresses mesenchymal marker expression in UUO kidney. A, Klotho mRNA levels determined by quantitative RT-PCR (qPCR). Right kidneys (UUO) and left kidneys (Control) from mice treated with vehicle (n = 5) and Klotho (0.01 mg/kg or 0.02 mg/kg, n = 5 for each dose) were compared on Day 3 and Day 7. Expression levels of mRNA were normalized with those in normal kidneys (Day 0). Data indicate means ± S.E. *, p < 0.05; **, p < 0.01 versus vehicle-treated mice at the same time points by two-tailed t test. #, p < 0.05 versus mice at Day 0 by two-tailed t test. B, αSMA mRNA levels. C, collagen-1 mRNA levels. D, TGF-β1 mRNA levels.
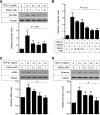
FIGURE 5.
Klotho protein inhibits TGF-β1 signaling and suppresses expression of mesenchymal markers in cultured cells. A, Klotho inhibits TGF-β1-induced phosphorylation of Smad2. NRK52E renal epithelial cells were incubated with secreted Klotho protein at the indicated doses for 30 min and then stimulated with TGF-β1 (10 ng/ml) for 30 min. Cell lysates were subjected to immunoblot analysis using antibody against phosphorylated Smad2 (pSmad2) or antibody that recognized Smad2 regardless of its phosphorylation state (Smad2). Typical results of 5 independent experiments are shown (upper panel). pSmad2/Smad2 ratios in each treatment were normalized with those without TGF-β1 and Klotho. Data indicate means ± S.E. of five independent experiments (lower panel). p = 0.02 by one-way ANOVA. B, Klotho inhibits TGF-β1-induced activation of a Smad-responsive reporter. HEK293 cells were transfected with a luciferase reporter containing Smad response elements (pGTCT2 × 2-Luc) and a lacZ expression vector for normalization. These cells were incubated with TGF-β1 and/or Klotho at the indicated doses and subjected to standard luciferase assays. Data indicate means ± S.E. of three independent experiments. p = 0.01 by one-way ANOVA. C, Klotho suppresses TGF-β1-induced increase in α-smooth muscle actin (αSMA) protein. NRK52E cells were incubated with Klotho protein and TGF-β1 at the indicated doses for 48 h in DMEM supplemented with 1% FBS. Cell lysates were subjected to immunoblot analysis using antibodies against αSMA and tubulin. A typical result of five independent experiments was shown (upper panel). The αSMA/tubulin ratios in each treatment were normalized with those without treatment. Data indicate means ± S.E. of five independent experiments (lower panel). *, p < 0.01 by one-way ANOVA. D, same as C, except that anti-Vimentin antibody was used.
FIGURE 6.
Klotho binds to TGFβR2 and inhibits TGF-β1 binding to the receptor. A, direct protein-protein interaction between Klotho and TGFβR2. Klotho protein was incubated with recombinant receptor ectodomain proteins with a human Fc tag and pulled down with protein A beads. The beads-bound protein was detected by immunoblot analysis using anti-Klotho (upper panel) or anti-human IgG (Fc, lower panel) antibodies. TGFβR2; Type-II TGF-β receptor, FGFR1b; fibroblast growth factor receptor 1α (IIIb), FGFR1c; fibroblast growth factor receptor 1α (IIIc), TGFβR1; Type-I TGF-β receptor, LRP-6; LDL receptor-related protein 6, EGFR; epidermal growth factor receptor, PDGFR; platelet-derived growth factor receptor-α. Klotho was reported to bind to FGFR1c but not to FGFR1b (6, 39), which served as positive and negative controls, respectively. B, Klotho inhibits TGF-β1 binding to TGFβR2 in vitro. TGF-β1 binding assays were performed using recombinant TGFβR2 protein on protein A beads in the presence of Klotho protein. Data indicate means of duplicated measurement and a fitting curve (_R_2 = 0.776). C, Klotho inhibits TGF-β1 binding to renal epithelial cells. TGF-β1 binding assay was performed in NRK52E cells in the presence of Klotho protein. Data indicate means ± S.E. (n = 4 for each dose). p = 0.017 by one-way ANOVA.
FIGURE 7.
Minimal additive effect of Klotho protein and neutralizing TGF-β1 antibody on mesenchymal marker expression in UUO kidney. UUO mice were treated with Klotho (0.02 mg/kg, intraperitoneal) or TGF-β1 antibody (1.5 mg/kg, intraperitoneal) or both every 48 h. On Day 7, mRNA levels of αSMA and collagen-1 in the right (UUO) and left (Control) kidneys were determined by qPCR. Data indicate means ± S.E. #, p < 0.05 versus mice without treatment by two-tailed t test.
FIGURE 8.
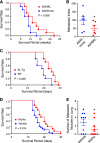
Klotho suppresses cancer metastasis and improve survival in mice. A, mice transplanted with A549KL cells (n = 10) survived longer than those transplanted with A549mock cells (n = 10). p = 0.038 by log-rank test. B, A549KL cells metastasize to the lung less efficiently than A549mock cells. A549KL and A549mock cells were labeled with different colors (GFP or RFP), mixed at 1:1 ratio, and transplanted into athymic mice (n = 6) by tail vein injection. Cells colonized in the lung were quantified by counting the number of colonies grown from the lung primary culture (metastatic index). Data indicate means ± S.E. *, p < 0.05 by two-tailed t test. C, Klotho-overexpressing transgenic mice (KL-Tg, n = 10) survived the 3LL transplantation longer than wild-type mice (WT, n = 10). p = 0.008 by log-rank test. D, Klotho protein injection improved survival of 3LL-transplanted mice. Wild-type mice were transplanted with 3LL cells by tail vein injection and then treated with Klotho protein (0.01 mg/kg, every 48 h, n = 14) or vehicle (n = 27). p = 0.014 by log-rank test. E, athymic nude mice were transplanted with 3LL cells by subcutaneous injection and then treated with Klotho protein (0.01 mg/kg, intraperitoneal, every 48 h, n = 10) or vehicle (n = 10) for 10 days. Lungs were harvested 3 weeks after transplantation to count the number of metastatic nodules. *, p < 0.05 by one-tailed t test with Welch's correction.
FIGURE 9.
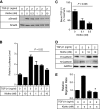
Secreted Klotho inhibits EMT in A549 cancer cells. A, Klotho inhibits TGF-β1-induced phosphorylation of Smad3 in A549 cells. A549 cells were incubated with secreted Klotho protein for 15 min and then stimulated with TGF-β1 for 15 min at the indicated doses. Cell lysates were subjected to immunoblot analyses using antibody against phosphorylated Smad3 (pSmad3) or antibody that recognized Smad3 regardless of its phosphorylation state (Smad3). B, Klotho inhibits TGF-β1-induced activation of the Smad-responsive reporter. A549 cells were transfected with a luciferase reporter containing Smad response elements (pGTCT2 × 2-Luc) and a lacZ expression vector for normalization. These cells were incubated with TGF-β1 or Klotho at the indicated doses for 6 h and subjected to a standard luciferase assay. The luciferase activity was normalized with that of non-treated cells. Data indicate means ± S.E. of three independent experiments. p = 0.02 by one-way ANOVA. C, Klotho inhibits TGF-β1 binding to A549 cells. TGF-β1 binding assays were performed in A549 cells in the absence or presence of Klotho protein (0.1 or 0.3 n
m
). The amount of bound TGF-β1 was normalized with that without Klotho. Data indicate means ± S.E. of four independent experiments. p = 0.006 by one-way ANOVA. D, Klotho protein suppresses TGF-β1-induced decrease in E-cadherin and increase in N-cadherin. A549 cells were treated with TGF-β1 and/or Klotho at the indicated doses for 6 h and then subjected to immunoblot analyses 48 h later. E, Klotho protein suppresses TGF-β1-induced cell migration. A549 cells were treated with TGF-β1 and/or Klotho at the indicated doses for 6 h and then subjected to a standard Transwell migration assay. Data indicate means ± S.E. of three independent experiments. *, p < 0.001 versus TGF-β1 treatment alone by two-tailed t test.
Similar articles
- MicroRNA-34a Promotes Renal Fibrosis by Downregulation of Klotho in Tubular Epithelial Cells.
Liu Y, Bi X, Xiong J, Han W, Xiao T, Xu X, Yang K, Liu C, Jiang W, He T, Yu Y, Li Y, Zhang J, Zhang B, Zhao J. Liu Y, et al. Mol Ther. 2019 May 8;27(5):1051-1065. doi: 10.1016/j.ymthe.2019.02.009. Epub 2019 Feb 15. Mol Ther. 2019. PMID: 30853453 Free PMC article. - Ginsenoside-Rg1 Protects against Renal Fibrosis by Regulating the Klotho/TGF-β1/Smad Signaling Pathway in Rats with Obstructive Nephropathy.
Li SS, He AL, Deng ZY, Liu QF. Li SS, et al. Biol Pharm Bull. 2018;41(4):585-591. doi: 10.1248/bpb.b17-00934. Biol Pharm Bull. 2018. PMID: 29607931 - Transforming growth factor-β1-mediated renal fibrosis is dependent on the regulation of transforming growth factor receptor 1 expression by let-7b.
Wang B, Jha JC, Hagiwara S, McClelland AD, Jandeleit-Dahm K, Thomas MC, Cooper ME, Kantharidis P. Wang B, et al. Kidney Int. 2014 Feb;85(2):352-61. doi: 10.1038/ki.2013.372. Epub 2013 Oct 2. Kidney Int. 2014. PMID: 24088962 - Two faces of TGF-beta1 in breast cancer.
Zarzynska JM. Zarzynska JM. Mediators Inflamm. 2014;2014:141747. doi: 10.1155/2014/141747. Epub 2014 May 7. Mediators Inflamm. 2014. PMID: 24891760 Free PMC article. Review. - Epithelial-mesenchymal plasticity in kidney fibrosis.
Hadpech S, Thongboonkerd V. Hadpech S, et al. Genesis. 2024 Feb;62(1):e23529. doi: 10.1002/dvg.23529. Epub 2023 Jun 22. Genesis. 2024. PMID: 37345818 Review.
Cited by
- Epigenetic reactivation of p21CIP1/WAF1 and KLOTHO by a combination of bioactive dietary supplements is partially ERα-dependent in ERα-negative human breast cancer cells.
Sinha S, Shukla S, Khan S, Tollefsbol TO, Meeran SM. Sinha S, et al. Mol Cell Endocrinol. 2015 May 5;406:102-14. doi: 10.1016/j.mce.2015.02.020. Epub 2015 Feb 25. Mol Cell Endocrinol. 2015. PMID: 25725373 Free PMC article. - Establishing a prognostic model of ferroptosis- and immune-related signatures in kidney cancer: A study based on TCGA and ICGC databases.
Han Z, Wang H, Long J, Qiu Y, Xing XL. Han Z, et al. Front Oncol. 2022 Aug 26;12:931383. doi: 10.3389/fonc.2022.931383. eCollection 2022. Front Oncol. 2022. PMID: 36091132 Free PMC article. - A contemporary atlas of the mouse diaphragm: myogenicity, vascularity, and the Pax3 connection.
Stuelsatz P, Keire P, Almuly R, Yablonka-Reuveni Z. Stuelsatz P, et al. J Histochem Cytochem. 2012 Sep;60(9):638-57. doi: 10.1369/0022155412452417. Epub 2012 Jun 21. J Histochem Cytochem. 2012. PMID: 22723526 Free PMC article. - Klotho and chronic kidney disease.
Hu MC, Kuro-o M, Moe OW. Hu MC, et al. Contrib Nephrol. 2013;180:47-63. doi: 10.1159/000346778. Epub 2013 May 3. Contrib Nephrol. 2013. PMID: 23652549 Free PMC article. Review. - Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling.
Zhou L, Li Y, Zhou D, Tan RJ, Liu Y. Zhou L, et al. J Am Soc Nephrol. 2013 Apr;24(5):771-85. doi: 10.1681/ASN.2012080865. Epub 2013 Apr 4. J Am Soc Nephrol. 2013. PMID: 23559584 Free PMC article.
References
- Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., Iwasaki H., Iida A., Shiraki-Iida T., Nishikawa S., Nagai R., Nabeshima Y. I. (1997) Nature 390, 45–51 - PubMed
- Imura A., Iwano A., Tohyama O., Tsuji Y., Nozaki K., Hashimoto N., Fujimori T., Nabeshima Y. (2004) FEBS Lett. 565, 143–147 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21EB009147/EB/NIBIB NIH HHS/United States
- R01CA129011/CA/NCI NIH HHS/United States
- P41 RR002584/RR/NCRR NIH HHS/United States
- R01 CA102792/CA/NCI NIH HHS/United States
- R01AG019712/AG/NIA NIH HHS/United States
- R01 AG019712/AG/NIA NIH HHS/United States
- U24 CA126608-05/CA/NCI NIH HHS/United States
- P30 CA142543/CA/NCI NIH HHS/United States
- R21 EB009147/EB/NIBIB NIH HHS/United States
- U24 CA126608-04/CA/NCI NIH HHS/United States
- U24 CA126608/CA/NCI NIH HHS/United States
- R01 CA139217/CA/NCI NIH HHS/United States
- R01CA102792/CA/NCI NIH HHS/United States
- R01 CA129011/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous