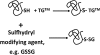S-glutathionylation: from molecular mechanisms to health outcomes - PubMed (original) (raw)
Review
S-glutathionylation: from molecular mechanisms to health outcomes
Ying Xiong et al. Antioxid Redox Signal. 2011.
Abstract
Redox homeostasis governs a number of critical cellular processes. In turn, imbalances in pathways that control oxidative and reductive conditions have been linked to a number of human disease pathologies, particularly those associated with aging. Reduced glutathione is the most prevalent biological thiol and plays a crucial role in maintaining a reduced intracellular environment. Exposure to reactive oxygen or nitrogen species is causatively linked to the disease pathologies associated with redox imbalance. In particular, reactive oxygen species can differentially oxidize certain cysteine residues in target proteins and the reversible process of S-glutathionylation may mitigate or mediate the damage. This post-translational modification adds a tripeptide and a net negative charge that can lead to distinct structural and functional changes in the target protein. Because it is reversible, S-glutathionylation has the potential to act as a biological switch and to be integral in a number of critical oxidative signaling events. The present review provides a comprehensive account of how the S-glutathionylation cycle influences protein structure/function and cellular regulatory events, and how these may impact on human diseases. By understanding the components of this cycle, there should be opportunities to intervene in stress- and aging-related pathologies, perhaps through prevention and diagnostic and therapeutic platforms.
Figures
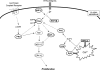
FIG. 1.
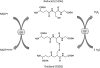
Glutathione as a biological redox buffer. The ratio of GSH/GSSG reflects the redox capacity of the cell. The ratio is kept in balance through oxidation/reduction reactions involving GSH peroxidase and GSH reductase. Reactive oxygen species-/reactive nitrogen species-induced changes that decrease GSH lead to cell death via apoptosis or necrosis. GP, GSH peroxidase; GR, GSH reductase; GSH, reduced glutathione; GSSG, oxidized glutathione.
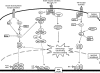
FIG. 2.
Regulation of GSH metabolism by GGT. GGT hydrolyzes extracellular GSH and releases glutamic acid and cysteinyl-glycine ○1. Cysteinyl-glycine is cleaved by the membrane-bound dipeptidase to cysteine and glycine, and the products are transported into the cells ○2. The γ-glutamyl moiety is transferred to the acceptors and transported into the cells ○3. The metabolic components participate in the de novo GSH synthesis catalyzed by GCS and GCL ○4. Intracellular GSH is exported out of cells through MRP ○5. The dashed lines indicate the upregulation of GCS, GCL, GGT, and MRP by oxidative stress. GCL, glutamate cysteine ligase; GCS, γ-glutamylcysteine synthetase; GGT, gamma-glutamyltransferase; MRP, multidrug resistant protein.
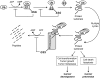
FIG. 3.
S-glutathionylation cycle. Cysteine residues on proteins that have a low pKa are targets for redox modulation under conditions of oxidative or nitrosative stress. The cysteine residue within proteins can be oxidized to form sulfenic, sulfinic, and sulfonic acids. Both sulfenic and sulfinic acids of proteins can be reduced or conjugated to GSH to form S-glutathionylated proteins via glutathione S-transferases, Grx, or nonenzymatically. The post-translational modification can be reversed by Grx and/or sulfiredoxin. Grx, glutaredoxin.
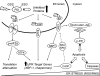
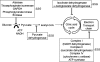
FIG. 4.
Biotin labeling of sulfhydryls to detect P-SSG. Labeling of S-glutathionylated proteins as described by Lind et al. (157). Free sulfhydryls are alkylated with _N_-ethylmaleimide followed by reduction of S-glutathionylated proteins with Grx3. Reduced SH groups are labeled with biotin-maleimide and purified with streptavidin agarose. Purified proteins are separated on a two-dimensional sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel, and spots are excised and digested with trypsin. The resultant tryptic peptides are spotted on a MALDI plate and subjected to MALDI-TOF mass spectrometry followed by database searching using the MASCOT algorithm. 2D SDS-PAGE, two-dimensional sodium dodecyl sulfate–polyacrylamide gel electrophoresis. MALDI-TOF, matrix-assisted laser desorption/ionization time-of-flight. (To see this illustration in color the reader is referred to the web version of this article at
).
FIG. 5.
Fluorescent detection of modified sulfhydryls. The thiol reactive compound ThioGlo-1 binds to free sulfhydryls on cysteine residues and can be used to semiquantitatively determine modified cysteines by fluorescence.
FIG. 6.
The interplay of protein phosphorylation and protein S-glutationylation pathways in cell signaling. Under basal conditions, GSTP forms heterodimers with JNK and TRAF2, resulting in kinase inactivation. Oxidative or nitrosative stress induces complex dissociation and results in S-glutathionylation and/or phosphorylation and activation of c-jun, JNK, and TRAF2. S-glutathionylation of GSTP leads to oligomerization and enzyme inactivation. These processes are reversed when stress is removed. JNK, c-Jun N-terminal kinase, GSTP, glutathione S-transferase pi. (To see this illustration in color the reader is referred to the web version of this article at
).
FIG. 7.
S-glutathionylation as a regulator for Ras-mitogen-activated protein kinase pathways in cancer. This figure depicts several sites in the Ras-mitogen-activated protein kinase pathway in cancer cells where reversible glutathionylation may serve as a potential regulatory mechanism. Ras, PKA, PKC, SHP-1, SHP-2, PTP1B, SERCA, RyR, and S100 proteins are highlighted, indicating that they are the targets of this modification.
FIG. 8.
Apoptosis/survival signaling pathways that are regulated by reversible S - glutathionylation. This figure demonstrates key signal transduction pathways in which regulation by reversible S-glutathionylation of signaling intermediates has been implicated. Kinases (PKC, IKK, JNK, and MEKK1), phosphatases (phosphatase and tensin homolog deleted from chromosome 10, and protein phosphatase 2A), transcription factors (p53, nuclear factor kappa B, and c-Jun), redox proteins (GSTP, Trx, and Prx), and death molecules (Fas and caspase 3) are highlighted, indicating that they are the targets of this modification. PP2A, protein phosphatase 2A; PTEN, phosphatase and tensin homolog deleted from chromosome 10.
FIG. 9.
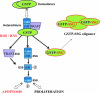
S-glutathionylation in the ubiquitin-proteosome pathway. This diagram outlines the sequential steps involved in ubiquitination/proteasome-mediated protein degradation. S-glutathionylation steps for E1, E2, and proteasome (-SSG) have been indicated. This redox-regulated post-translational modification leading to inhibition of protein degradation could differentially influence cancer development and cancer prevention, depending on the function of the substrate protein in specific signaling pathways.
FIG. 10.
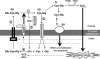
The UPR and pro-apoptotic pathways. This figure depicts the UPR signaling cascades and UPR-related pro-apoptotic pathways. During homeostasis, three endoplasmic reticulum membrane signaling molecules, pancreatic ER kinase, IRE1, and ATF6, are negatively regulated through associations with BiP. Oxidative (reactive oxygen species) and nitrosative (reactive nitrogen species) stress leads to S-glutathionylation of PDI that blunts isomerase activity. As a consequence, protein folding is dysregulated and leads to the accumulation of unfolded proteins. BiP triggers the UPR by disassociating from the membrane signaling molecules, thereby promoting transcriptional and translational regulation of gene expression and signals pro-apoptotic pathways. ATF6, activating transcription factor 6; ER, endoplasmic reticulum; PERK, pancreatic ER kinase; UPR, unfolded protein response.
FIG. 11.
Enzymes involved in energy metabolism that are regulated by S-glutathionylation. This figure summarizes the key participants in energy metabolism, of which regulation by reversible S-glutathionylation (-SSG) has been implicated. The steps of glycolysis, citric acid cycle, and electron transport chain are indicated.
FIG. 12.
Glutathione synthesis in neurons and astrocytes. Both neurons and astrocytes are able to synthesize glutathione through γ-GCS and glutathione synthetase, whereas only astrocytes are able to export GSH. Extracellular GSH can be broken down and the constitutive amino acids (glutamate, cysteine, and glycine) taken up by neurons for subsequent GSH synthesis. In addition, astrocytes are able to exchange glutamate for cystine through the cystine-glutamate exchange of system xc-, which allows cystine to be used for GSH synthesis. Further, it has been shown that GSH can act as a neurotransmitter through binding to NMDA, AMPA, or even GSH receptors. (To see this illustration in color the reader is referred to the web version of this article at
).
Similar articles
- S-glutathionylation, friend or foe in cardiovascular health and disease.
Rashdan NA, Shrestha B, Pattillo CB. Rashdan NA, et al. Redox Biol. 2020 Oct;37:101693. doi: 10.1016/j.redox.2020.101693. Epub 2020 Aug 22. Redox Biol. 2020. PMID: 32912836 Free PMC article. Review. - Evaluating the Oxidative Stress in Renal Diseases: What Is the Role for S-Glutathionylation?
Tamma G, Valenti G. Tamma G, et al. Antioxid Redox Signal. 2016 Jul 20;25(3):147-64. doi: 10.1089/ars.2016.6656. Epub 2016 Apr 19. Antioxid Redox Signal. 2016. PMID: 26972776 Review. - Protein Thiol Redox Signaling in Monocytes and Macrophages.
Short JD, Downs K, Tavakoli S, Asmis R. Short JD, et al. Antioxid Redox Signal. 2016 Nov 20;25(15):816-835. doi: 10.1089/ars.2016.6697. Epub 2016 Jul 13. Antioxid Redox Signal. 2016. PMID: 27288099 Free PMC article. Review. - Posttranslational modification of cysteine in redox signaling and oxidative stress: Focus on s-glutathionylation.
Mieyal JJ, Chock PB. Mieyal JJ, et al. Antioxid Redox Signal. 2012 Mar 15;16(6):471-5. doi: 10.1089/ars.2011.4454. Epub 2012 Jan 4. Antioxid Redox Signal. 2012. PMID: 22136616 Free PMC article. - Molecular mechanisms and clinical implications of reversible protein S-glutathionylation.
Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Mieyal JJ, et al. Antioxid Redox Signal. 2008 Nov;10(11):1941-88. doi: 10.1089/ars.2008.2089. Antioxid Redox Signal. 2008. PMID: 18774901 Free PMC article. Review.
Cited by
- Beneficial and Detrimental Effects of Reactive Oxygen Species on Lifespan: A Comprehensive Review of Comparative and Experimental Studies.
Shields HJ, Traa A, Van Raamsdonk JM. Shields HJ, et al. Front Cell Dev Biol. 2021 Feb 11;9:628157. doi: 10.3389/fcell.2021.628157. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33644065 Free PMC article. Review. - S-glutathionylation, friend or foe in cardiovascular health and disease.
Rashdan NA, Shrestha B, Pattillo CB. Rashdan NA, et al. Redox Biol. 2020 Oct;37:101693. doi: 10.1016/j.redox.2020.101693. Epub 2020 Aug 22. Redox Biol. 2020. PMID: 32912836 Free PMC article. Review. - Emerging mechanisms of glutathione-dependent chemistry in biology and disease.
Janssen-Heininger YM, Nolin JD, Hoffman SM, van der Velden JL, Tully JE, Lahue KG, Abdalla ST, Chapman DG, Reynaert NL, van der Vliet A, Anathy V. Janssen-Heininger YM, et al. J Cell Biochem. 2013 Sep;114(9):1962-8. doi: 10.1002/jcb.24551. J Cell Biochem. 2013. PMID: 23554102 Free PMC article. Review. - Mechanistic link between erectile dysfunction and systemic endothelial dysfunction in type 2 diabetic rats.
Musicki B, Hannan JL, Lagoda G, Bivalacqua TJ, Burnett AL. Musicki B, et al. Andrology. 2016 Sep;4(5):977-83. doi: 10.1111/andr.12218. Epub 2016 May 6. Andrology. 2016. PMID: 27153512 Free PMC article. - Strain-dependent glutathionylation of fibronectin fibers impacts mechano-chemical behavior and primes an integrin switch.
Li W, Moretti L, Su X, Yeh CR, Torres MP, Barker TH. Li W, et al. Nat Commun. 2024 Oct 9;15(1):8751. doi: 10.1038/s41467-024-52742-3. Nat Commun. 2024. PMID: 39384749 Free PMC article.
References
- Adachi T. Pimentel DR. Heibeck T. Hou X. Lee YJ. Jiang B. Ido Y. Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279:29857–29862. - PubMed
- Adachi T. Weisbrod RM. Pimentel DR. Ying J. Sharov VS. Schoneich C. Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources